Abstract
Two-pore channels (TPCs) are related to voltage-gated Ca2+ and Na+ channels. They most likely work as dimers with each of the two TPC protein subunits containing two pore-forming domains. Recent studies suggest that TPCs are expressed on the membranes of endosomes and lysosomes where they form receptors for nicotinic acid adenine dinucleotide phosphate (NAADP), the most potent Ca2+ mobilizing messenger inside cells. Upon activation by NAADP, Ca2+ release from endolysosomal stores through TPCs triggers cytoplasmic Ca2+ signals. Because of discrete localizations of these acidic vesicles and their small, albeit variable, sizes, the Ca2+ signals from endolysosomes are local and, perhaps, represent unique elementary Ca2+ events. These localized signals can be converted into regenerative global Ca2+ waves by triggering Ca2+-induced Ca2+ release from endoplasmic reticulum. We will discuss the implication of these findings and the significance of TPCs in integrative Ca2+ signaling in animal cells.
Ca2+ Mobilizing Messengers in Animal Cells
Calcium ions play a pivotal role in cell signaling. To this end, cells maintain a low intracellular Ca2+ concentration ([Ca2+]i) of ∼100 nM at rest, with increases in [Ca2+]i regulating a wide range of cellular events, including contraction, secretion and programmed cell death. [Ca2+]i increases are brought about by Ca2+ influx from the extracellular space and/or Ca2+ release from intracellular Ca2+ stores. Traditionally, the sarco/endoplasmic reticulum (S/ER) has been considered to be the major, releasable intracellular Ca2+ store. From this store, Ca2+ may be released through the opening of inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) and/or ryanodine receptors (RyRs), the two groups of intracellular Ca2+ release channels located on S/ER membranes. Of the recognized Ca2+ mobilizing second messengers, IP3 and cyclic ADP ribose (cADPR) facilitate this process by activating IP3Rs and RyRs, respectively. In recent years, however, a growing body of evidence has suggested an important role for acidic organelles (i.e., endosomes, lysosomes, as well as secretory vesicles) in the regulation of Ca2+ signaling and nicotinic acid adenine dinucleotide phosphate (NAADP) has been identified as being of primary importance to the mobilization of these stores. NAADP is in fact the most potent of the known Ca2+ mobilizing messengers, being effective at low nanomolar concentrations,Citation1 yet until recently the molecular identity of the NAADP receptor has remained a mystery.
Two-Pore Channels and their Relation to Other Cation Channels
In a recent study, we presented the first evidence that two-pore channels (TPCs or TPCNs for gene names) are located on endolysosomal membranes in mammalian cells and demonstrated that they bind NAADP and mediate NAADP-dependent Ca2+ release from acidic organelles.Citation2 TPCs are novel members of the voltagegated cation channel superfamily, which is composed of more than 140 structurally and functionally diverse members. These range from the inwardly rectifying K+ channels that contain as few as two transmembrane (TM) segments to CaV and NaV channels that contain 24 TM segments in the pore-forming subunits.Citation3 The four-fold symmetry of the latter group suggests two rounds of duplication from an ancestral 6-TM architecture, which represents >50% of the members of this superfamily including voltage-gated and Ca2+-activated K+ channels, cyclic nucleotidegated channels, cation channels of spermatozoa (CatSpers), and all transient receptor potential (TRP) channels. These channels work as tetramers of the 6-TM subunits, where the fifth and sixth TM α helices (S5 and S6) and, between these, a membrane reentrant pore loop (P-loop), together form the central pore for ions to pass through.
The M1 and M2 TM segments of inwardly rectifying K+ channels are structural and functional analogs of the S5 and S6 helices of the 6-TM channels. Two of these 2-TM units are linked to form the two-pore K+ channels. TPCs are, however, different from two-pore K+ channels in that they are formed by links between two 6-TM units; hence each TPC protein contains a total of 12 putative TM α helices.
Based on the knowledge that the single pore-domain 6-TM channels work as tetramers and the four pore-domain CaV and NaV channels only require one pore-forming subunit, TPCs are predicted to function as dimers.Citation4 Pair-wise comparison using primary sequences at the TM regions suggests that TPCs are most related to CaV and NaV channels and to a slightly lesser degree CatSpers and the class II TRP channels such as TRPP2 and TRPML1. Therefore, it is possible that TPCs represent an intermediate evolutionary step, i.e., the first round duplication, from single pore-domain 6-TM channels to four pore-domain CaV and NaV channels. The presence of an array of positively charged residues separated by two hydrophobic ones at the putative S4 segment of TPCs is consistent with this notion and further suggests some voltage dependence, similar to their four-pore domain cousins. However, the number of positively charged residues in the S4 segments of TPCs is much less than those found in CaV and NaV channels, suggesting, perhaps, relatively weak voltage dependence. The higher homology to Ca2+, Na+, and non-selective cation channels than to K+ channels also suggests that TPCs are most likely Ca2+ permeable or at least cation nonselective.
Three non-allelic TPCN genes (TPCN1, TPCN2 and TPCN3) are found in most vertebrate species as well as in sea urchins. It is important to note that studies on sea urchin eggs have been vital to much of what we have learned about NAADP-dependent Ca2+ signaling to date. Both the discovery of NAADP as a Ca2+ mobilizing messenger and the demonstration of its action at acidic stores were initially made using sea urchin egg samples.Citation5–Citation7 Therefore, it is comforting to know that all three TPCN genes are present in sea urchins. By contrast, a search of human or chimp genome revealed only about one-third of the TPCN3 sequence and in rats and mice the TPCN3 gene is completely missing, suggesting that TPC3 is not present in these mammalian species. Moreover, in many land plants there exists a single TPC gene that is equally distant from the three mammalian genes. Nonetheless, the presence of TPC genes in both animal and plant kingdoms suggests that this is a rather ancient channel family.
Being widespread in all vertebrates and perhaps all deuterostomes, TPC genes are not always found in protostomes. For example, the genomes of commonly used model species, C. elegans and Drosophila melanogaster, do not appear to contain a homologous sequence to the TPCs, except for those that have been identified as NaV or CaV channels. Indeed, TPCN genes may be lost in all flies and mosquitoes, but TPCN1 is preserved in other insects, such as honeybees and silkworms. Arachnids (e.g., ticks) may have both TPCN1 and TPCN3 while flatworms (e.g., Schistosoma mansoni) have TPCN2. Importantly, sequences for all three TPCNs are found in trichoplax and sea anemones and those for TPCN1 and TPCN3 are present in choanoflagellates, indicating that multiple TPCN genes had appeared early on in the evolution of the animal kingdom, even though their loss in certain species suggests that TPCs are not essential for life.
Two-Pore Channels are Ca2+ Release Channels of Acidic Organelles
Despite the suggested intermediate role in the evolution of fourpore domain channels, there has been no functional demonstration of TPC channel activity on the plasma membrane. Several years ago, however, Arabidopsis TPC was shown to form slow vacuolar channels involved in Ca2+-dependent Ca2+ release in plant vacuoles.Citation8 Consistent with this finding, we demonstrated that mammalian TPCs are predominantly expressed in the membranes of endolysosomes. Specifically, TPC1 and TPC3 are mainly present on different populations of endosomes, while TPC2 is targeted to lysosomes.Citation2 Thus, in both plant and animal cells, TPCs are targeted to acidic stores rather than to the plasma membrane. Importantly, we showed for the first time that membranes enriched in TPC2 contain both high (∼5 nM) and low (∼10 µM) affinity NAADP binding sites consistent with previous studies on endogenous NAADP binding membranes derived from a variety of cell types. Furthermore, we showed that NAADP-evoked Ca2+ release was greatly enhanced by overexpression of TPC2 and markedly attenuated by knockdown of TPC2 expression. Specifically, we measured changes in [Ca2+]i in HEK293 cells in response to either flash photolysis of caged- NAADP or intracellular dialysis of known concentrations of NAADP. With both protocols, wild type cells showed very small and highly localized Ca2+ transients whereas cells stably overexpressing human TPC2 displayed robust, global Ca2+ transients in response to NAADP. Furthermore, high (mM) concentrations of NAADP precipitated homologuous self-inactivation/desensitization of this release process in a manner consistent with previous studies on NAADP-dependent Ca2+ signaling in wild-type cells. We concluded, therefore, that TPCs represent a family of NAADP receptors. Using similar approaches, two other groups have subsequently reported data that, in principle, support our conclusion.Citation4,Citation9
Two-Pore Channels Generate Elementary Ca2+ Signals that can be Converted to Global Ca2+ Waves through Coupling to S/ER Ca2+ Release
Interestingly, biphasic Ca2+ transients are evoked by NAADP in HEK293 cells that stably overexpress TPC2, with an initial slow pacemaker phase followed by a large secondary Ca2+ transient. We further demonstrated that the initial phase of intracellular Ca2+ transients represents Ca2+ mobilization from acidic stores while the secondary phase resulted from Ca2+ release from ER stores via IP3Rs. Thus, both phases of Ca2+ release were blocked by depletion of lysosomal Ca2+ stores with bafilomycin A1, a vacuolar proton pump inhibitor that disrupts the proton gradient necessary for acidic stores to remain replete in Ca2+. In marked contrast, only the secondary, global Ca2+ transient was abolished following depletion of ER Ca2+ stores with thapsigargin or by inhibiting IP3Rs with heparin. This observation suggests that NAADP-induced Ca2+ signals in HEK293 cells play a triggering role for ER Ca2+ release, an idea that is not new because crosstalk between NAADP-induced Ca2+ release and that mediated by IP3Rs and RyRs has been well documented in a number of cell systems.Citation10–Citation17 Such coupling is believed to occur through Ca2+-induced Ca2+ release (CICR), a well-known property of RyRs, but also clearly documented for IP3Rs.Citation18 For the latter, CICR may require some basal IP3 levels and in each case a threshold Ca2+ concentration may have to be met at either the cytoplasmic, the ER luminal side, or both. Only in the presence of a robust CICR mechanism, is it possible that a relatively small quantity of Ca2+ release from acidic stores in response to NAADP may be subsequently amplified via the S/ER into a marked and global Ca2+ wave. Thus, it is interesting to note that the NAADP-dependent Ca2+ signals presented by Brailoiu and co-workers as evidence of NAADP-dependent signaling via TPC1, do not exhibit an identifiable “pacemaker” phase of Ca2+ release and are relatively weakly attenuated without any change in waveform when ER Ca2+ release is blocked.Citation9 By contrast, in their studies on TPC2, Zong and co-workers observed only very slow and prolonged increases in [Ca2+]i that appeared entirely independent of ER Ca2+ release.Citation4
It is clear that the coupling efficiency between NAADP-induced endolysosomal Ca2+ signals and S/ER Ca2+ release will be dependent on a number of factors. First, unlike the large network that is the S/ER, endolysosomes generally constitute relatively small, discrete and mobile vesicles. The amplitude of the Ca2+ signal arising from an endosome or lysosome will therefore be limited by the Ca2+ content of a given vesicle. Furthermore, because NAADP receptors are not sensitized by Ca2+,Citation19,Citation20 the Ca2+ signal generated by release solely from the acidic store is not inherently regenerative. This constitutes a mechanism of release that is quite different from that driven by IP3R or RyR activation.Citation21–Citation23 Thus, in isolation NAADP-evoked Ca2+ transients are composed of scattered, local events (possibly of different sizes) that are dependent on the distribution and Ca2+ content of the acidic stores as well as the local NAADP concentration. Clustering in time and space of the affected acidic stores may therefore be necessary in order to breach a given threshold for CICR from the S/ER. Second, the NAADP-sensitive stores need to be situated very close to the S/ER in order to achieve efficient coupling. Indeed, in some vascular smooth muscle cells the space between the RyR-containing SR and a subpopulation of lysosomes may be less than 100 nm. These tight junctions may constitute a “trigger zone” for the initiation of propagating Ca2+ signals in response to NAADP.Citation17,Citation24 Third, the subtypes of RyRs and IP3Rs are also important. For example, among the three RyR types, RyR3 may be preferentially targeted to lysosome-SR junctions in pulmonary arterial smooth muscle cells and likely plays a specialized role in converting local NAADP-evoked Ca2+ signals into regenerative, global Ca2+ transients.Citation24 It is also important to note, therefore, that differences in Ca2+ sensitivity exist between the three IP3R subtypes.Citation18 Thus, the relative distribution of these receptors in different cell types and their respective cellular locations may also impact on the coupling efficiency between endolysosome stores and the S/ER. Fourth, if the coupling is mainly dependent on IP3Rs, the basal IP3 levels, which are likely not uniform throughout the cell, could prove decisive. Finally, both IP3R and RyRs are regulated by Ca2+ in the S/ER lumen.Citation25–Citation27 Thus, Ca2+ arising from the acidic organelles may also affect the S/ER release probability after its uptake into the S/ER lumen via SERCA pumps.
HEK293 cells do not express functional RyRs,Citation28 leaving IP3Rs to function as the major ER Ca2+ release channels. In the case of wild type HEK293 cells that do not support global Ca2+ signals in response to NAADP (up to 1 µM), it would appear that the elementary Ca2+ signals evoked by NAADP through the endogenous TPCs are insufficient to breach the Ca2+ threshold required for activating IP3Rs. In our studies, this barrier to efficient coupling appears to be overcome by the stable overexpression of TPC2, which allows for this threshold to be easily breached. Any delay in triggering the secondary phase of ER Ca2+ release may therefore be due to the time required for temporal and spatial summation of the Ca2+ signals emanating from individual vesicular stores that contribute to the collective.
It is therefore interesting to note that previous studies have identified stimulus-evoked Ca2+ transients in Hela cells that also consist of a pacemaker phase composed of elementary Ca2+ signals of varying sizes and secondary, global Ca2+ waves. Here too, the pacemaker phase can be abortive (non-regenerative) or regenerative depending on the recruitment of the elementary Ca2+ signals in frequency, amplitude and spatial domains.Citation22,Citation23,Citation29 Despite the presumption that the varying sizes of the elementary Ca2+ signals arose from the opening of either single IP3Rs or RyRs, or a cluster of these receptors, the phenotypes of the [Ca2+] changes are rather similar to what we observed in HEK293 cells stimulated by NAADP. Arguably, while the roles for IP3Rs or RyRs in generating elementary Ca2+ signals have been unequivocally established in some studies, neither the nature of the store nor the type of the Ca2+ mobilizing messenger was thoroughly examined in many other cases. Therefore, we propose that a significant portion of the elementary Ca2+ events described during the onset of stimulus-evoked Ca2+ transients in many cell types may have arisen from acidic stores via TPCs and not necessarily via IP3Rs or RyRs, as was presumed (). Thus, the variable sizes of endolysosomes, or even the variable Ca2+ conductance of each TPC may perhaps explain at least some of the different elementary Ca2+ signals that have been given various names, such as: blips, quarks, puffs, bumps, sparks and etc.Citation29
Admittedly, the mechanism of NAADP production is not completely understood, but at least for some transmitters, e.g., cholecystokinin (CCK) and endothelin-1, it may involve the same receptors that generate IP3.Citation10,Citation17,Citation30 Given this fact, it is quite possible that weak receptor stimulation may produce a sufficient amount of NAADP to activate TPCs but not enough IP3 to directly trigger ER Ca2+ release through IP3Rs. However, it has also been suggested that the CCK-A receptors have two affinities for CCK with the activation of the high affinity site producing NAADP and cADPR and that of the low affinity site generating IP3.Citation31 Of course, there are other identified stimuli that may generate NAADP alone. For example, glucose and glucagon-like peptide 1 induce NAADP production in pancreatic β-cells without necessarily generating IP3,Citation32,Citation33 and although ADPR cyclases, such as CD38, are most frequently studied, there may be other pathways or enzymes that underpin NAADP synthesis in different mammalian cells.Citation33–Citation35
Biological Significance of Two-Pore Channels
There are many questions that remain about the biological significance of the NAADP/TPC pathway. If the main function of the NAADP/TPC pathway is to determine whether a particular stimulus should become abortive or regenerative depending on the summation of elementary events in frequency, amplitude and spatial domains, then it must play an important computational and/or filtering role for environmental stimuli. As such, it may be essential for some stimuli but dispensable for others. For example, in pancreatic acinar cells, Ca2+ oscillations evoked by CCK are dependent on NAADP while those induced by bombesin or acetylcholine are not.Citation31,Citation32,Citation36 In accordance with this, only CCK, but not acetylcholine, causes transient NAADP production lasting less than 60 seconds in these cells.Citation37 Furthermore, other endolysosome-localized Ca2+-permeable channels, e.g., TRPML1, TRPML3, TRPM1 and TRPM2,Citation38–Citation40 may contribute to elementary Ca2+ signals from acidic stores as well. Among them, TRPML1 and TRPM2 have been proposed to serve functions in response to NAADP,Citation40,Citation41 although others have suggested that this may not be their primary function.Citation38,Citation42–Citation44
On the other hand, the NAADP/TPC signaling pathway appears to be equally able to function as a discrete process with the consequences of its activity on occasion being entirely independent of ER coupling. For instance, in pancreatic β-cells, an elevation of extracellular glucose concentration triggers NAADP production and Ca2+ release from acidic organelles.Citation32,Citation45 It is likely that the elementary Ca2+ signals from the acidic stores directly gate Ca2+-activated cation channels located on the plasma membrane,Citation2 resulting in membrane depolarization and consequent Ca2+ influx through voltage-gated Ca2+ channels (green pathway in ). That this NAADP-evoked cation current is lacking in β-cells prepared from TPC2 null mice,Citation2 demonstrates a critical role for TPC2 in this response. Yet, it is not clear whether IP3Rs and RyRs still play a significant role in the overall Ca2+ signals induced by high glucose. Amplification of the initial NAADP-induced Ca2+ signals by the ER is possible in the β-cells such that in the case of an insulin-induced increase in [Ca2+]i it requires IP3Rs,Citation46 while in relation to glucagon-like peptide-1 evoked Ca2+ signals it may depend on RyRs.Citation33 However, in these studies, Ca2+ influx from the extracellular space appears to be critical either for the entire Ca2+ transient,Citation33 or just the secondary phase,Citation46 implicating still a major role for the plasma membrane Ca2+ channels.
Other functions that require calcium release from endolysosomes include trafficking and fusion of endolysosomal vesicles as well as functional regulation of endolysosome enzymes, perhaps through consequent pH changes in the acidic stores.Citation47–Citation50 It is yet to be explored to what extent each of the TPCs exerts a role in these functions.
To date, a number of genetic studies have linked the TPCN2 gene to pigmentation of skin cells, hearing defects and cancer (reviewed in Citationref. 51), but the mechanisms involved remain to be elucidated. Moreover, this list will likely expand as NAADP has been shown to play important roles in a wide spectrum of physiological functions from insulin and digestive enzyme secretion,Citation10,Citation45 smooth muscle contraction,Citation16,Citation17,Citation30 T cell activation,Citation15 to neurite outgrowth.Citation52 Therefore, further investigation of the NAADP/TPC pathway in Ca2+ signaling should provide not only a better understanding of the generation and function of diverse spatiotemporal patterns of [Ca2+]i changes in different cell types but will also aid in our understanding of the signaling mechanisms that underlie normal cellular physiology and the pathogenesis of certain human diseases.
Abbreviations
| [Ca2+]i | = | intracellular Ca2+ concentration |
| cADPR | = | cyclic ADP ribose |
| CCK | = | cholecystokinin |
| CICR | = | Ca2+-induced Ca2+ |
| IP3 | = | inositol 1,4,5-trisphosphate |
| IP3R | = | IP3 receptor |
| NAADP | = | nicotinic acid adenine dinucleotide phosphate |
| RyR | = | ryanodine receptor |
| S/ER | = | sarco/endoplasmic reticulum |
| TM | = | transmembrane |
| TPC | = | two-pore channel |
| TRP | = | transient receptor potential |
Figures and Tables
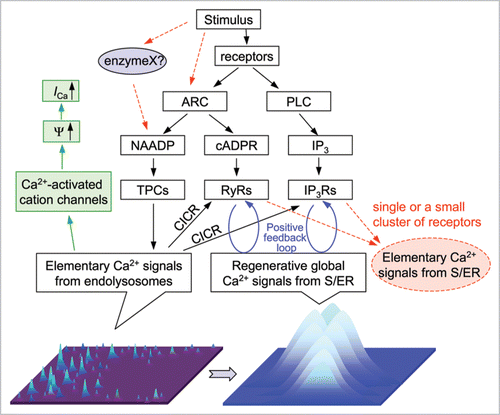
Figure 1 The proposed role for two-pore channels in integrative Ca2+ signaling. Flow chart shows pathways for NAADP, cADPR and IP3 production and their effects on intracellular Ca2+. The drawings at the bottom depict elementary Ca2+ signals generated by TPCs (left, lighter color and higher position for higher Ca2+ concentrations) and global Ca2+ signals generated by activating IP3Rs and/or RyRs (right). Red dashed lines indicate alternative pathways. The green pathway on the left shows an alternative consequence of TPC-mediated Ca2+ release. ARC, ADP-ribose cyclase, including CD38, PLC, phospholipase C, Ψ, membrane potential. ICa, Ca2+ current through plasma membrane.
Acknowledgements
Supported by grants from US National Institutes of Health (GM081658 and DK081654 to M.X.Z.; AG028856, AG028614, HL069000 and CA095739 to J.M.), the British Heart Foundation (CF/05/050 to A.M.E.) and the Wellcome Trust (070772 to A.M.E.; 07925 4/Z/06/Z and 084101/Z/07/Z to J.P. and A.G.).
References
- Galione A, Petersen OH. The NAADP receptor: new receptors or new regulation?. Mol Interv 2005; 5:73 - 79
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009; 459:596 - 600
- Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltagegated ion channel superfamily. Pharmacol Rev 2005; 57:387 - 395
- Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, et al. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch 2009; 458:891 - 899
- Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem 1995; 270:2152 - 2157
- Chini EN, Beers KW, Dousa TP. Nicotinate adenine dinucleotide phosphate (NAADP) triggers a specific calcium release system in sea urchin eggs. J Biol Chem 1995; 270:3216 - 3223
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 2002; 111:703 - 708
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, et al. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 2005; 434:404 - 408
- Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol 2009; 186:201 - 209
- Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature 1999; 398:74 - 76
- Cancela JM, Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. Two different but converging messenger pathways to intracellular Ca2+ release: the roles of nicotinic acid adenine dinucleotide phosphate, cyclic ADP-ribose and inositol trisphosphate. EMBO J 2000; 19:2549 - 2557
- Churchill GC, Galione A. Spatial control of Ca2+ signaling by nicotinic acid adenine dinucleotide phosphate diffusion and gradients. J Biol Chem 2000; 275:38687 - 38692
- Churchill GC, Galione A. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO J 2001; 20:2666 - 2671
- Santella L, Kyozuka K, Genazzani AA, De Riso L, Carafoli E. Nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release. Interactions among distinct Ca2+ mobilizing mechanisms in starfish oocytes. J Biol Chem 2000; 275:8301 - 8306
- Berg I, Potter BV, Mayr GW, Guse AH. Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca2+-signaling. J Cell Biol 2000; 150:581 - 588
- Boittin FX, Galione A, Evans AM. Nicotinic acid adenine dinucleotide phosphate mediates Ca2+ signals and contraction in arterial smooth muscle via a two-pool mechanism. Circ Res 2002; 91:1168 - 1175
- Kinnear NP, Boittin FX, Thomas JM, Galione A, Evans AM. Lysosome-sarcoplasmic reticulum junctions. A trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J Biol Chem 2004; 279:54319 - 54326
- Bootman MD, Lipp P. Ringing changes to the ‘bellshaped curve’. Curr Biol 1999; 9:876 - 878
- Genazzani AA, Galione A. Nicotinic acid-adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem J 1996; 315:721 - 725
- Chini EN, Dousa TP. Nicotinate-adenine dinucleotide phosphate-induced Ca2+-release does not behave as a Ca2+-induced Ca2+-release system. Biochem J 1996; 316:709 - 711
- Bootman MD, Berridge MJ. Subcellular Ca2+ signals underlying waves and graded responses in HeLa cells. Curr Biol 1996; 6:855 - 865
- Bootman MD, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell 1997; 91:367 - 373
- Bootman M, Niggli E, Berridge M, Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J Physiol 1997; 499:307 - 314
- Kinnear NP, Wyatt CN, Clark JH, Calcraft PJ, Fleischer S, Jeyakumar LH, et al. Lysosomes colocalize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium 2008; 44:190 - 201
- Irvine RF. ‘Quantal’ Ca2+ release and the control of Ca2+ entry by inositol phosphates—a possible mechanism. FEBS Lett 1990; 263:5 - 9
- Missiaen L, Taylor CW, Berridge MJ. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature 1991; 352:241 - 244
- Sitsapesan R, Williams AJ. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by luminal Ca2+. J Membr Biol 1994; 137:215 - 226
- Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, et al. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci 2004; 117:2813 - 2825
- Bootman MD. Hormone-evoked subcellular Ca2+ signals in HeLa cells. Cell Calcium 1996; 20:97 - 104
- Zhang F, Zhang G, Zhang AY, Koeberl MJ, Wallander E, Li PL. Production of NAADP and its role in Ca2+ mobilization associated with lysosomes in coronary arterial myocytes. Am J Physiol Heart Circ Physiol 2006; 291:274 - 282
- Cancela JM. Specific Ca2+ signaling evoked by cholecystokinin and acetylcholine: the roles of NAADP, cADPR and IP3. Annu Rev Physiol 2001; 63:99 - 117
- Yamasaki M, Masgrau R, Morgan AJ, Churchill GC, Patel S, Ashcroft SJ, et al. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem 2004; 279:7234 - 7240
- Kim BJ, Park KH, Yim CY, Takasawa S, Okamoto H, Im MJ, et al. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes 2008; 57:868 - 878
- Soares S, Thompson M, White T, Isbell A, Yamasaki M, Prakash Y, et al. NAADP as a second messenger: neither CD38 nor base-exchange reaction are necessary for in vivo generation of NAADP in myometrial cells. Am J Physiol Cell Physiol 2007; 292:227 - 239
- Vasudevan SR, Galione A, Churchill GC. Sperm express a Ca2+-regulated NAADP synthase. Biochem J 2008; 411:63 - 70
- Burdakov D, Galione A. Two neuropeptides recruit different messenger pathways to evoke Ca2+ signals in the same cell. Curr Biol 2000; 10:993 - 996
- Yamasaki M, Thomas JM, Churchill GC, Garnham C, Lewis AM, Cancela JM, et al. Role of NAADP and cADPR in the induction and maintenance of agonist-evoked Ca2+ spiking in mouse pancreatic acinar cells. Curr Biol 2005; 15:874 - 878
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 2008; 455:992 - 996
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal 2009; 2:21
- Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal 2009; 2:23
- Zhang F, Li PL. Reconstitution and characterization of a nicotinic acid adenine dinucleotide phosphate (NAADP)-sensitive Ca2+ release channel from liver lysosomes of rats. J Biol Chem 2007; 282:25259 - 25269
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001; 411:595 - 599
- Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 2001; 293:1327 - 30
- Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, et al. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem 2006; 281:7294 - 7301
- Masgrau R, Churchill GC, Morgan AJ, Ashcroft SJ, Galione A. NAADP: a new second messenger for glucose-induced Ca2+ responses in clonal pancreatic beta cells. Curr Biol 2003; 13:247 - 251
- Johnson JD, Misler S. Nicotinic acid-adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human beta cells. Proc Natl Acad Sci USA 2002; 99:14566 - 14571
- Mueller OT, Rosenberg A. β-Glucoside hydrolase activity of normal and glucosylceramidotic cultured human skin fibroblasts. J Biol Chem 1977; 252:825 - 829
- Morgan AJ, Galione A. NAADP induces pH changes in the lumen of acidic Ca2+ stores. Biochem J 2007; 402:301 - 310
- Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med 2008; 14:1247 - 1255
- Luzio JP, Bright NA, Pryor PR. The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans 2007; 35:1088 - 1091
- Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, et al. The acid test: the discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca2+ release channels. Pflugers Arch 2009; 458:869 - 876
- Brailoiu E, Churamani D, Pandey V, Brailoiu GC, Tuluc F, Patel S, et al. Messenger-specific role for nicotinic acid adenine dinucleotide phosphate in neuronal differentiation. J Biol Chem 2006; 281:15923 - 15928