Abstract
It was recently discovered that the photoreceptor cryptochrome is involved in mediating magnetosensitive entrainment of the internal clock of fruit flies (1). This discovery follows other recent studies implicating a role of cryptochrome in mediating magnetic sensitivity in orientation responses of fruit flies (2,3) and growth responses of plants (4). Such widespread use of the same molecule for mediating magnetic sensitivity might suggest that cryptochrome is in some way optimal for detecting the magnetic field of the earth and that the magnetoreception function cannot be easily taken over by other molecules. This raises the question what properties might set cryptochromes apart from other molecules in terms of their ability to detect the geomagnetic field. Here, we will discuss possible answers to this question. We will first review the likely biophysical mechanism by which magnetic fields can be detected by a photoreceptor and discuss what constitutes an optimal photo-magneto-receptor. We will then discuss in how far cryptochrome matches the profile of an optimal molecule and what further steps are required for more conclusive answers.
The geomagnetic field is weak and requires specialized sensors to be detected: in biological systems, the effects of the geomagnetic field will likely be masked by thermal fluctuations and other noise. One way to increase the strength of geomagnetic field effects is to use materials with a strong magnetic moment, such as iron oxides. In the proper geometry, iron oxide clusters can behave like a compass needle and magnetic bacteria use such a compass needle mechanism to find up and down. An alternative possibility is to use short-lived, specialized photochemical reactions, for which thermal noise does not have time to effectively mask effects of magnetic fields.
In the photochemical mechanism, on which we will focus here, absorption of light triggers an electron transfer from a donor to an acceptor molecule (confer ), thus creating a donor-acceptor pair with one unpaired electron each, a so-called radical pair. This radical pair lives up to tens of microseconds before decaying into reaction products. The two electrons on the donor and acceptor radicals possess a quantum mechanical property, the electron spin, that can be thought of as a small magnetic moment. Unlike a compass needle that aligns its direction with that of the local magnetic field and then stops moving, the electrons’ magnetic moments never come to rest, but move perpetually in a fashion comparable to that of a table top, spinning or precessing around the axis of the local magnetic field. The local magnetic field at the position of the electron spins is composed of the external (geomagnetic) field and the, generally stronger, internal magnetic field created by magnetic moments of hydrogen and nitrogen nuclei. The relative alignment of the two electron magnetic moments at any given time is denoted as the spin state of the radical pair and is a critical determinant for their chemical reactivity. Depending on the spin state, different reaction products will be formed, and at different rates. In essence, the intermediate radical pair state acts like a switch that governs the balance between two product states. Even a small geomagnetic field can shift the balance between the product states, increasing the concentration of one and decreasing the concentration of the other. If the internal magnetic fields are anisotropic, then the effect of the geomagnetic field depends not only on its intensity, but also on its direction, thereby potentially creating a magnetic compass.Citation5 The physical chemistry of this mechanism is well understoodCitation6 and one can even design molecules that are sensitive to earth-strength magnetic fields via this mechanism.Citation7
While the photochemical mechanism may appear more farfetched than the idea of a compass needle, its biological realization may, in fact, be comparatively straightforward. Unlike a compass needle mechanism, the photochemical mechanism does not require any specialized iron oxide material, nor a procedure to embed iron oxide clusters in membranes so as to prevent aggregation. Instead, the photochemical mechanism only requires pigment molecules that transfer electrons upon light absorption. Such molecules are, of course, readily available even in the oldest photochemical reactions, as the primary photosynthetic step constitutes a radical-pair reaction, and magnetic fields can indeed affect photosynthetic reactions under certain conditions.Citation8 Another class of pigments capable of electron transfers are flavins, the active group in cryptochromes, phototropins and other flavoproteins. Certainly, not every pigment undergoing electron transfer reactions is sensitive to earth-strength magnetic fields and we will discuss below what aspects will likely be needed to endow such sensitivity. A functioning compass needle of iron oxides needs to be have dimensions of several micrometers in length and will require a multi-cellular context for an effective force transduction mechanism, whereas many copies of cryptochromes or other pigment-protein complexes can be embedded within a single cell. Finally, if the pigment-protein complex in question is in fact a photoreceptor, then magnetic sensing can be perceived as an indirect effect on light sensing of this receptor, without the need of developing a novel signal transduction pathway.
Cryptochromes appear to be a particularly intriguing candidate for photo-magnetoreceptors.Citation5 First, they have clearly been shown to act as photoreceptors in a variety of organisms. In plants, they serve as photosensors for numerous developmental and growth responses such as hypocotyl growth and leaf expansion, induction of flowering time, entrainment of the circadian clock and as regulators of gene expression. In animals such as Drosophila, cryptochromes can be involved directly as light inputs into the circadian clock.Citation9 Cryptochromes are fairly ubiquitous and have been found in several of the organisms for which magnetic field effects were demonstrated, including fruit flies, plants and migratory birds. Most importantly, the photochemical properties of cryptochromes show several features that are favorable for detection of weak magnetic fields (confer ). Firstly, cryptochromes are activated via an intraprotein electron transfer mechanism that generates radical pairs comprising flavin and one of several tryptophan and/or tyrosine residues forming a chain of electron transfer to the surface of the protein. Citation10–Citation12 Radical formation in this way activates the protein and induces biological activity. Any physiological or external factor that increases the lifetime of the radical will necessarily result in an increased cryptochrome signal at a given light intensity, and any factor that tends to decrease the lifetime of the radical will result in reduced cryptochrome activity at the same photon fluence (light intensity). In addition to the forward light dependent (photoreduction) mechanism that activates cryptochromes, they also undergo a reverse reaction (reoxidation) that restores the fully oxidized (inactive resting) form of cryptochromes in the dark (). This reoxidation reaction occurs by a mechanism that could also generate radical pairs (superoxide and/or peroxide radicals, flavin radicals) and therefore be magnetically sensitive. For example, if applied magnetic fields have an effect on the rate of the reoxidation reaction they would decrease or increase the overall lifetime of the active form of cryptochrome and therefore affect biological activity. In sum, cryptochromes photochemically offer an almost ideal paradigm for magnetosensitivity as even a subtle shift in a redox equilibrium would be perceptible as an alteration in photoreceptor response in the organism.
Experimental evidence supports such a magnetosensing role for cryptochrome. In the plant system, application of weak magnetic fields to Arabidopsis thaliana seedlings indeed causes an enhanced cryptochrome response,Citation4 whereby the plants respond as if they have been exposed to higher intensities of blue light than was in fact the case. These results are consistent with the proposed mechanism of either an increased efficiency in the forward reaction or reduced efficiency of the reverse (dark reversion) reaction of cryptochrome under conditions of applied magnetic field. The physiological responses measured in the exposed plants were inhibition of hypocotyl elongation and anthocyanin accumulation in response to blue light, and their enhanced cryptochrome responsivity was manifested as shortened hypocotyls and increased anthocyanin accumulation as compared to control plants grown under identical light intensities. Although no directional information is conveyed in the plant system, it is a unique example of magnetic field effects in a biological organism mediated by a sensor whose primary purpose is light response. The photochemical reaction mechanism provides an explanation of how such sensitivity to weak magnetic fields can arise serendipitously in photoreceptors undergoing oxidation/reduction steps during activation.
In the case of Drosophila cryptochromes, a similar effect appears to hold.Citation1 For the circadian clock, cryptochrome serves to lengthen the period at limiting light intensity, just prior to the onset of arrhythmia. Under conditions of applied magnetic field, the period length of the clock is significantly lengthened for the majority of the flies, indicating enhanced cry signal in these flies even though photon fluence (light intensity) is the same. Therefore, application of applied magnetic field in this system mimics the effect of increased blue light signal intensity on cryptocchrome. Cry mutant or knockout flies showed no magnetic field sensitivity, wherease flies overexpressing cryptochrome showed enhanced sensitivity as compared to wild type. These results provide support for the plant experiments showing that application of magnetic field indeed mimics increased light signal strength from the viewpoint of the photoreceptor. A further recent report investigating the effect of applied magnetic field on a behavioral response of Drosophila, namely the ability to orient towards a food source, has also implicated cryptochrome.Citation2 In these experiments, flies were trained to associate applied magnetic field with a food source, and learned to use this signal for orientation. The behavioral response also required cryptochrome since it was absent in cry mutant flies. However, although the outcome of these trials involved orientation, the actual physiological effect on the fly is consistent with an intensity sensing effect such as found for plants and for the circadian rhythm effect. Flies could have correlated enhanced (brighter) blue light caused by the applied magnetic field with the food stimulus.
One of the best studied magnetoreception models is that of migratory birds.Citation13 Here, magnetic orientation experiments allowed for more detailed exploration of the mechanism underlying magnetoreception. The magnetic compass of migratory birds depends on the wavelength and intensity of ambient light,Citation14,Citation15 as expected in a radical-pair mechanism, but it is unclear which photoreceptor or combination of photoreceptors mediates these light effects.Citation16 Monitoring the effect of adding artificial magnetic fields specifically designed to disrupt a photochemical mechanism provides a means to test for the mechanism underlying magnetic orientation. Oscillating fields with frequencies in the MHz range were shown to disrupt orientation of migratory birds at specific frequencies and intensities in a manner consistent with expectations from a photochemical mechanism.Citation17–Citation20
Such effects were also seen on magnetic orientation in several species of birds,Citation20 but not in orientation behavior of mole rats,Citation21 nor in orientation responses of birds unrelated to migratory homing, Citation22,Citation23 indicating a common magnetoreception mechanism in some but not all animals and responses. Although there is clear evidence for the involvement of a radical pair mechanism in the magnetosense of many migrating birds, it is unclear which photoreceptors mediate these light effects.Citation16 Cryptochromes have been found in the eyes of birds,Citation24,Citation25 and some genetic hints exist for their involvement in magnetoreception,Citation26 but the lack of transgenic birds has precluded more clear-cut evidence so far.
Many open questions remain at present. The identity of the radical pair that may be responsible for cryptochrome magnetic sensitivity is unknown. The lifetime of the trp and tyr radicals involved in forward electron transfer is on the order of milliseconds, whereas the flavin radical can last for minutes under physiological conditions.Citation27 Although these possibilities appear well suited to eventual magnetic sensitivity, a recent study suggestsCitation19 that at one of the radicals involved in the bird magnetosensitivy may not be covalently bound to the protein. This could implicate possible additional radicals whose identity remains to be determined. For instance, the nature of the electron donor at the surface of the protein is unknown and there may be a relatively long lived radical formed at the terminal step where the surface tryptophan or tyrosine is reduced by solvent. Furthermore, it is possible that there may formation of radicals during the dark reversion reaction involving oxygen or superoxide radicals.Citation19 These pathways need to be additionally explored. Theoretically, modeling needs to proceed from proof-of-principle models to more realistic models including structural, physical and chemical details.
In summary, molecular biology and biophysical studies indicate long-lived radical pairs in cryptochrome proteinCitation10,Citation27 and related pigment-protein complexes,Citation28 and recent intriguing results show magnetic field effects on the forward electron transfer reaction in photolyases, which are closely related to cryptochromes. Citation29 Genetic studies show absence of magnetic field effects when cryptochromes are deleted, but one yet needs to show that introducing cryptochromes into a system creates magnetic sensitivity, ideally with a hint of the evolutionary advantage of such sensitivity. The small size of magnetic field effects suggest that we yet have to find physiological responses for which the magnetic field provide the dominant, and not incident stimuli. Given the relatively short time from the first suggestion of cryptochrome as a magnetoreceptor in 2000,Citation5 the amount of studies from different fields supporting the photo-magnetoreceptor and cryptochrome hypotheses, briefly reviewed here, is promising. It suggests that we may be only one step away from a true smoking gun revealing the long-sought after molecular nature of receptors underlying the 6th sense and thus the solution of a great outstanding riddle of sensory biology.
Figures and Tables
Figure 1 Illustration of the photochemical magnetic detection mechanism. A light-induced electron transfer creates an intermediate donor-acceptor pair (D + A) with separate electron spins (dotted arrows). The electron spins move in their local magnetic fields, thereby changing their relative orientation, e.g., from an anti-parallel to a parallel orientation. The relative orientation of the spins, denoted as singlet or triplet spin states, governs what products can be formed and at what rate. Importantly, even a small geomagnetic field (blue) alters the spin motion in a different way on the donor than on the acceptor, in relation to the internal magnetic fields (orange) in both molecules. This results in a change of the singlet to product ratio, as indicated by the blue circles.
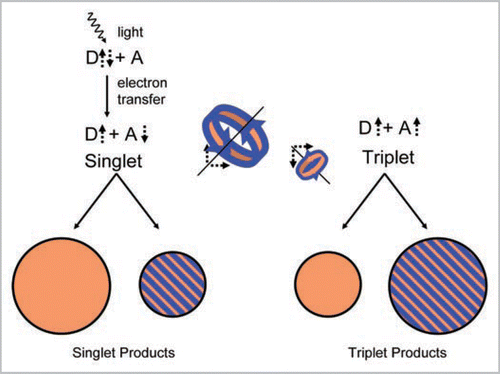
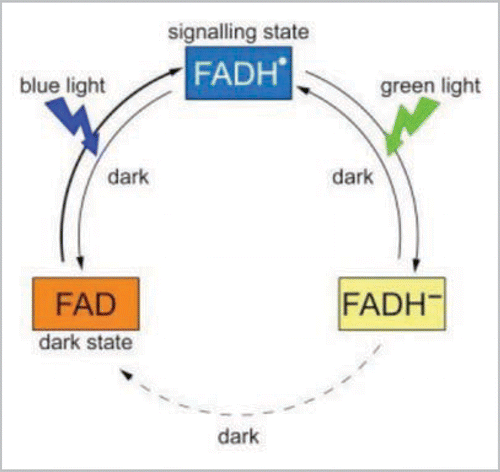
Figure 2 Photocycle of plant cryptochromes. Cryptochromes are bound to a light-absorbing flavin cofactor (FAD) which can exist in three interconvertable redox forms: (FAD, FADHℴ, FADH−). In the dark, cryptochrome is found with flavin in the oxidized state (FAD) which, upon irradiation, undergoes reduction to the FADHℴ form. This reaction requires both electron and proton transfer from nearby amino acid residues in the protein, generating amino acid radicals in turn, which are ultimately reduced through a chain of electron transfer to the protein surface. Further illumination of the flavin radical (by blue or green light) results in formation of the fully reduced FADH− form that is biologically inactive. In the dark, the flavin is spontaneously re-oxidized to restore the fully oxidized resting state through a mechanism that is as yet poorly characterized but involves molecular oxygen and may give rise to superoxide radicals. Therefore both forward and reverse reactions may involve the formation of radical pairs. The degree of cryptochrome activation is governed by the equilibrium reached between the competing forward and reverse reactions under conditions of constant illumination.
References
- Yoshii T, Ahmad M, Helfrich-Forster C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biology 2009; 7:813 - 819
- Gegear RJ, Casselman A, Waddell S, Reppert SM. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 2008; 454:1014 - 1016
- Dommer DH, Gazzolo PJ, Painter MS, Phillips JB. Magnetic compass orientation by larval Drosophila melanogaster. J Insect Physiol 2008; 54:719 - 726
- Ahmad M, Galland G, Ritz T, Wiltschko R, Wiltschko W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 2007; 225:615 - 624
- Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J 2000; 78:707 - 718
- Rodgers CT. Magnetic field effects in chemical systems. Pure and Applied Chem 2009; 81:19 - 43
- Maeda K, Henbest KB, Cintolesi F, Kuprov I, Rodgers CT, Liddell PA, et al. Chemical compass model of avian magnetoreception. Nature 2008; 453:387 - 390
- Boxer SG, Chidsey CED, Roelofs MG. Magnetic field effects on reaction yields in the solid-state—an example from photosynthetic reaction centers. Ann Rev Phys Chem 1983; 34:389 - 417
- Emery P, Stanewsky R, Helfrich-Förster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron 2000; 26:493 - 504
- Giovani B, Byrdin M, Ahmad M, Brettel K. Lightinduced electron transfer in a cryptochrome blue-light photoreceptor. Nat Struct Biol 2003; 10:489 - 490
- Bouly JP, Schleicher E, Dionisio-Sese M, Vandenbussche F, Van Der Straeten D, Bakrim N, et al. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J Biol Chem 2007; 282:9383 - 9391
- Hoang N, Schleicher E, Kacprzak S, Bouly JP, Picot M, Wu W, et al. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol 2008; 6:160
- Wiltschko R, Wiltschko W. Magnetic orientation in animals 1995; Berlin Springer,
- Wiltschko R, Wiltschko W. Magnetoreception. Bioessays 2006; 28:157 - 168
- Muheim R, Bäckman J, Akesson S. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J Exp Biol 2002; 205:3845 - 3856
- Johnsen S, Mattern E, Ritz T. Light-dependent magnetoreception: quantum catches and opponency mechanisms of possible photosensitive molecules. J Exp Biol 2007; 210:3171 - 3178
- Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W. Resonance effects indicate radical pair mechanism for avian magnetic compass. Nature 2004; 429:177 - 180
- Thalau P, Ritz T, Stapput K, Wiltschko R, Wiltschko W. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwiss 2005; 92:86 - 90
- Ritz T, Wiltschko R, Hore PJ, Rodgers CT, Stapput K, Thalau P, et al. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys J 2009; 96:3451 - 3457
- Wiltschko W, Freire R, Munro U, Ritz T, Rogers L, Thalau P, et al. The magnetic compass of domestic chickens, Gallus gallus. J Exp Biol 2007; 210:2300 - 2310
- Thalau P, Ritz T, Burda H, Wegner RE, Wiltschko R. The magnetic compass mechanisms of birds and rodents are based on different physical principles. J Roy Soc Interface 2006; 3:583 - 587
- Wiltschko R, Ritz T, Stapput K, Thalau P, Wiltschko W. Two different types of light-dependent responses to magnetic fields in birds. Curr Biol 2005; 15:1518 - 1523
- Wiltschko R, Stapput K, Ritz T, Thalau P, Wiltschko W. Magnetoreception in birds: different physical processes for two types of directional responses. HFSP Journal 2007; 1:41 - 48
- Mouritsen H, Janssen-Bienhold U, Liedvogel M, Feenders G, Stalleicken J, Dirks P, et al. Cryptochromes and activity markers co-localize in bird retina during magnetic Orientation. Proc Natl Acad Sci USA 2004; 101:14294 - 14299
- Möller A, Sagasser S, Wiltschko W, Schierwater B. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften 2 2004; 91:585 - 588
- Freire R, Munro U, Rogers LJ, Sagasser S, Wiltschko R, Wiltschko W. Different responses in two strains of chickens (Gallus gallus) in a magnetic orientation test. Anim Cogn 2008; 11:547 - 552
- Biskup T, Schleicher E, Okafuji A, Link G, Hitomi K, Getzoff ED, et al. Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor. Angew Chem 2009; 48:404 - 407
- Liedvogel M, Maeda K, Henbest K, Schleicher E, Simon T, Timmel CR, et al. Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE 2007; 2:1106
- Henbest KB, Maeda K, Hore PJ, Joshi M, Bacher A, Bittl R, et al. Magnetic-field effect on the photoactivation reaction of Escherichia coli DNA photolyase. Proc Natl Acad Sci USA 2008; 105:14395 - 14399