Abstract
Protein kinases mediate most of the signal transduction in eukaryotic cells, controlling important cellular processes. Functioning as sensors and switches, kinases play a critical role in the regulation of cell fate decisions: proliferation, differentiation or death. Cellular sensors must have signaling properties well suited for the processing and propagation of external or internal stimuli that promote irreversible processes. These properties include ultrasensitivity, hysteresis and digital responses. Ultrasensitivity means to produce a very large response to a small increase in stimulus after a threshold is crossed, hysteresis (a form of biochemical memory) means sustained activation when the stimulus has disappeared, and digital is an all-or-none response at a single cell level. These properties are present in JNK, a stress protein kinase that regulates cell death. In a recent article, we have characterized Xenopus AMPK, a stress protein kinase that controls energy levels in the cell, showing that is regulated similar to the mammalian ortholog. By using Xenopus oocytes we studied the AMPK signaling system and compared to JNK. Our work showed that AMPK is ultrasensitive to an apoptotic stimulus (hyperosmolar sorbitol) but, in contrast to JNK, does not show hysteresis. By single cell analysis we found that the response of AMPK and JNK to hyperosmolar sorbitol is all-or-none (digital) in character, and that initial graded responses of both protein kinases are converted into digital during the critical period of cytochrome c release. We proposed a model to explain the cell death program as integration of multiple digital signals from stress sensors, that now I extend to a more general model for sensing, integrating and making choices in the cell and the organism.
More that 20 years ago, Tony Hunter proposed that protein kinases may be used in cellular regulatory circuits in a fashion analogous to transistors or chips in computers.Citation1 In electronic circuits transistors are used either as simple on/off switches or as amplifiers for an electric current. Transistors commonly have two inputs, which regulate current flow and gain, and a single output. Protein kinases have switching properties. For instance, Ca2+/calmodulin-dependent protein kinase II (CaMKII) has the properties of a molecular switch, becoming independent of the Ca2+ signal following Ca2+-stimulated autophosphorylation.Citation2 The elegant works of James E. Ferrell et al. showed that phosphorylation of MAPK or JNK can yield ultrasensitivity (reflected in a stimulus/response curve with a very steep upstroke), and that ultrasensitive systems embedded in a positive feedback loop have the potential to exhibit bistable behavior, switching between discrete stable steady states without being able to rest in intermediate states.Citation3–Citation5 The analogy with a transistor is particularly striking, as well pointed by D. Grahame Hardie years ago.Citation6 Given the switching properties of these enzymes one can build a sophisticated multidimensional regulatory network with exquisite selectivity and sensitivities using protein kinases at the nodes.Citation1 We can consider living cells as information-processing devices. The input information received in the cell from the internal and external environment would be integrated and processed into output responses or cellular choices (to proliferate, to differentiate or to die). Thus, a cellular circuitry based on protein kinases would integrate the variety of different inputs into a common output or cellular decision.
The most irreversible decision for a cell is to commit suicide. The cell must decide, in the face of noxious environmental stimuli, whether to survive and repair the damage or to die by apoptosis. Kinases may play an important role in the “sensing” phase of cell death, ultimately determining cell fate. Some kinases are directly activated, others are turned off, and still others are indirectly activated when cleaved by caspases.Citation7 Death could be determined by an integration of signals. The energy level in a cell/organism is an important signal and must be tightly regulated to perform multiple functions or cell death programs will otherwise be engaged. The AMP-activated protein kinase (AMPK) is an energy sensor and a homeostatic regulator of cellular ATP levels.Citation8 The c-Jun N-terminal kinase (JNK) is a stress protein kinase that regulates cell death.Citation9 JNK is activated in Xenopus oocytes by hyperosmolar sorbitol showing a bistable response.Citation4 The three hallmarks of a bistable system are: (1) strong ultrasensitivity, (2) all-or-none (digital) response at the individual cell level, and (3) sustained activation when the stimulus has disappeared (hysteresis). These properties may be important for the regulation of cell death. Xenopus oocytes are a suitable model to study these properties, as has been previously reported for JNK and MAPK cascades.Citation3–Citation5 In our recently published articleCitation10 we studied the AMPK signaling system, using JNK as a control. First, we characterized Xenopus AMPK (since nothing was previously described) showing that it was regulated in the oocytes similar to the mammalian ortholog, and making this system an ideal model in which to study the AMPK signaling properties. Our study showed that hyperosmolar sorbitol induced apoptosis in Xenopus oocytes and an ultrasensitive response of AMPK, whereas the response to antimycin (which did not produce apoptosis) was only slightly cooperative.Citation10 The response of AMPK and JNK to hyperosmolar sorbitol was digital at the level of individual oocytes whereas the response of AMPK to antymicin was graded. We also observed an important difference in the response of AMPK and JNK to hyperosmolar sorbitol: JNK did show hysteresis, as previously described, but AMPK did not (meaning that the activity returned to basal levels when the stimulus was not present). In other words, JNK is a bistable system whereas AMPK is a monostable signaling system. Importantly, experiments with individual oocytes allowed to conclude that initial graded responses of AMPK and JNK (2 h after treatment) were converted into digital with time (4 h after treatment), just when cytochrome c release takes place (between 2 h and 4 h, depending of the experiment). The digital response was not abrogated with the broad inhibitor of caspases Z-VAD.fmk, and AMPK and JNK responses were not completely correlated with cytochrome c release at the level of individual oocytes.
We can interpret these results in two ways: (1) AMPK and JNK are not involved in cytochrome c release and another unknown factor is responsible for cell death; or (2) AMPK or JNK digital responses are not sufficient to induce apoptosis but the combination of digital responses from different stress sensors can engage the cell death program (see ). I prefer the second hypothesis for several reasons: (1) Based in the literature, it would be very surprising that the rapid activation of AMPK and JNK (just a few minutes after hyperosmolar sorbitol treatment) will not have any effect in the regulation of cell death (another question is how: preventing or inducing), (2) When AMPK and JNK are “digitally” activated in the same cell there is a high probability to find cytochrome c release and caspase activation, and (3) Integration of different signals from different sensors seems logical in such an important and irreversible decision as cell death.
A relevant concept to understand the generation of digital responses in the cell is the signal thresholds levels, as depicted in our model (). Each protein kinase considered as a sensor, has specific threshold levels for its signal. A certain stimulus (in the range of intermediate doses) will produce certain level of activation. Surpassing the threshold level means to maintain the activation over time, whereas not achieving the threshold means that the initial response of the kinase became transitory. At a certain time (4 h in the figure) we will observe digital responses, if we analyze single cells. Of course, signaling thresholds for one specific kinase may vary from different cell types. A different concept in cell signaling is hysteresis: when the stimulus disappears some kinases will remain on (bistable systems), whereas others will switch off (monostable systems). This differential behavior can be important for the progression of the cell death program: bistable systems are irreversible switches and therefore could facilitate apoptosis. The combination of mono and bistable signaling systems may be useful to define the frontier between integration of the signals and cell decision, evaluating the strength and duration of the noxious stimuli.
How many protein kinases will show a digital response to a certain stimuli? This is difficult to predict, but I think that digital responses are widespread in cell signaling. I postulate that several protein kinases, in addition to AMPK and JNK, will show a digital response to hyperosmolar sorbitol (schematized as protein kinase X in ). We also must keep in mind that different stimuli can produce a different response for the same protein kinase. Thus, as we have described, the response of AMPK to hyperosmolar sorbitol is digital, whereas the response to antimycin is graded.Citation10 In addition to specific threshold levels for activation of digital kinases, we can also consider an “apoptosis threshold level” for the cell to engage the death program. Thus, the relative levels of proapoptotic and antiapoptotic molecules set a threshold that must be overcome for a given stimulus to irreversibly activate the cell death pathway. The basic idea is that the net of protein kinases digitally activated (or inhibited) can modify the pro/anti-apoptotic equilibrium (this is what we mean by integration of responses) pushing the cell to make a choice: to survive or to die. An example of such an integrative mechanism would be the activation of the ataxia telangiectasia mutated (ATM) protein kinase and p53. Single-cell response of p53 (an inducer of Mdm2) and Mdm2 (a negative regulator of p53) to ionizing radiation (IR) is oscillatory and digital. The number of oscillations depends on the radiation dose.Citation11 A model was proposed where ATM kinase, which is as a sensor of double strand, breaks in the DNA, acts as a switch activating the p53—Mdm2 oscillator module in a digital way.Citation12 Interestingly, the authors speculate that pro-apoptotic p53 target genes will be gradually integrated over multiple cycles of p53 pulses until they reach a certain threshold value that will activate apoptosis.
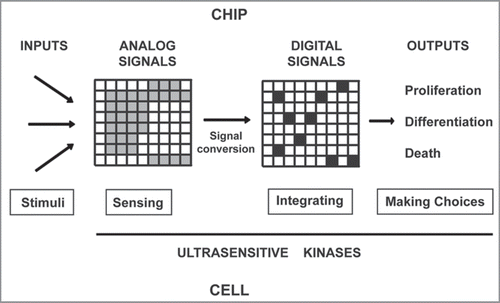
If we take again the analogy of cells as information devices and of protein kinases as transistors or chips in computers, we can imagine networks of kinases that generate digital information to “run” different programs (like the cell death program) (). Therefore, a general model for sensing, integrating and making choices, based in the generation of digital responses, may be applied to different biological processes. However, we can not discard cellular programs based in analog signals, depending of the stimulus. In fact, analog and digital programs could be rewired. For instance, MAP kinase modules are tightly controlled by positive and negative feedback loops, as well as a variety feed-forward loops. Different feedback control systems can theoretically allow the same module to generate different signal outputs, including graded (analog) or digital responses.Citation13 Thus, EGF generates an analog ERK activation in PC-12 cells (inducing cell proliferation), whereas NGF treatment produced a digital ERK output (required for differentiation). The authors found that PKC phosphorylation of Raf kinase inhibitory protein (RKIP) reconfigures the MAPK module from an analog system to digital one by a positive feedback loop mechanism, thereby directing opposing cell fate decisions.Citation14 Tian et al. have shown that activation of the MAPK module through Ras nanoclusters in the cell membrane generates high-fidelity analog output functioning as an analog-digitalanalog converter.Citation15 Recently, it has been described that Ras activation in lymphoid cells is characterized by digital signaling and hysteresis as a consequence of a positive feedback loop.Citation16 In principle, it could be possible to develop algorithms to predict outputs from specific inputs (signals) if we know all the pathways involved and the basic properties of the nodes (protein kinases) in the network. Can we extend this reasoning to the whole organism? Or at least, can we extend it to an important organ as the brain?
The majority of protein kinases are expressed in the brain. It seems possible that many of them show digital responses to physiological stimuli. Most studies of signal transduction use saturating doses of the stimuli and one can question whether the responses obtained are physiologically relevant. We observed digital responses of AMPK and JNK in single cells (oocytes) at intermediate doses of sorbitol. I propose that physiological concentrations of different stimuli in the brain can generate digital responses of protein kinases in neurons.
Now, I will consider a fundamental process in the brain: memory. Long-term potentiation (LTP) is an activity-dependent strengthening of synapses that is though to underlie memory storage. Interestingly, individual synapses appear to have all-or-none potentiation with different thresholds levels.Citation17 Therefore, it seems that synaptic memories at hippocampus are encoded in a digital manner. LTP is regulated by several protein kinases, including cAMP-dependent protein kinase (PKA), PKC, MAPK, CaMKIICitation18 and the atypical PKC isozyme protein kinase Mzeta (PKMζ).Citation19 Importantly, persistent protein kinase activity exists in LTP for CaMKIICitation20 and PKMζ.Citation21 A model of long term memory has been proposed based in the molecular switch properties of of CaMKII.Citation22 CaMKII is necessary for long-term potentiation (LTP) in the hippocampus. The kinase can be activated to different degrees and is able to function as a frequency detector. CaMKII acts as a bistable switch in the postsynaptic density, turning on when a threshold number of kinase sites are phosphorylated. The on state of the switch can last for very long periods because the kinase acts faster that the phoshatase (reviewed in ref. 23). Indeed, PKMζ, acting as a constitutive kinase, is necessary and sufficient for LTP maintenanceCitation19 and is required for several types of long-term memories.Citation24
Some years ago, a digital model was proposed for the process of memory and retrieval of the stored information (recall). It was postulated that spike trains (brief sequence of action potentials) generated by neurons are digital signals representing binary numbers (coded information) and are used by the brain to perform internal calculations.Citation25 Cesare Marchetti proposed that a spike train could be the way that a neuronal event becomes digitized, so that the memory of this event can be reactivated by a number carried by a spike train roaming the brain.Citation26 The same author also proposes that a spike train representing a digital number sets CaMKII into one of its 10Citation12 potential states (“freezing the information”), and later a spike train carrying the same number will reactivate it in terms of stimulating calmodulin to liberate Ca2+. A recall requires the simultaneous switching on of thousands of synapses by a signal of some sort that travels through the brain. Who and how starts the chain of events leading to a recall? Marchetti reasons that a single cell may hold the code of a single memory in the form of one or more molecules of coded CaMKII and would be able to initiate the spike train that will open the circuit.
A model for long term memory based in one or two protein kinases (CaMKII and PKMζ) is attractive, but taking into account that many protein kinases show digital responses we should consider an scenario where multiple digital signals are integrated: first at the level of single cells generating an output, and later as a net of cells that would generate a recall, or even a new idea as a result of mixed outputs. If this becomes true, to analyze the signaling events in single cells and the networks of digital responses generated in the brain could help to understand how we think and learn, and eventually how we take decisions.
Figures and Tables
Figure 1 A digital model for initiation of the cell death program. Single cell response to stress (hyperosmolar sorbitol) is ultrasensitive and graded for 2 h, and then a critical period starts where the activity achieved is compared with specific threshold levels for each protein kinase. Kinase activity under the threshold level returns to basal state, whereas activation over the threshold level remains high in the critical period. When we analyze individual oocytes at 4 h, a digital response is obtained, but at 2 h the response is analog. The cell would integrate multiple digital signals (PK X represents one or more protein kinases) for the critical period evaluating whether to trigger a cell death program.
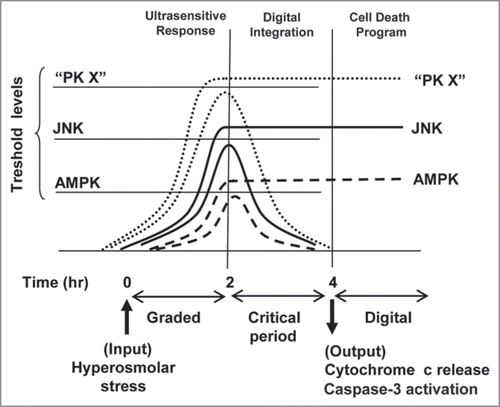
Figure 2 Life as a chip: A digital model for cell decisions. Different stimuli (inputs) are sensed by ultrasensitive protein kinases producing a plethora of analog signals, and some of them are converted into digital signals. The digital responses obtained by the network of kinases are integrated and defines a cellular program, which is translated into a cell decision (outputs). Thus, the generation of digital responses by protein kinases might be the basis for important biological processes: from development to memory (see text).
Acknowledgements
This work was supported by Grant PI 05/1748 from Fondo de Investigaciones Sanitarias (FIS). J.M.L. is recipient of a contract from the “Programa Ramón y Cajal” (MEC).
Addendum to:
References
- Hunter T. A thousand and one protein kinases. Cell 1987; 50:823 - 829
- Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell 1986; 44:861 - 870
- Ferrell JE Jr, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science 1998; 280:895 - 898
- Bagowski CP, Ferrell JE Jr. Bistability in the JNK cascade. Curr Biol 2001; 11:1176 - 1182
- Ferrell JE Jr.. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 2002; 14:140 - 148
- Hardie DG. Protein kinases at the frontier. Trends in Cell Biol 2001; 11:142
- Utz PJ, Anderson P. Life and death decisions: regulation of apoptosis by proteolysis of signaling molecules. Cell Death Differ 2000; 7:589 - 602
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 2007; 8:774 - 785
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol 2007; 19:142 - 149
- Martiáñez T, Francès S, López JM. Generation of digital responses in stress sensors. J Biol Chem 2009; 284:23902 - 23911
- Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet 2004; 36:147 - 150
- Ma L, Wagner J, Rice JJ, Hu W, Levine AJ, Stolovitzky GA. A plausible model for the digital response of p53 to DNA damage. Proc Natl Acad Sci USA 2005; 102:14266 - 14271
- Harding A, Hancock JF. Ras nanoclusters: combining digital and analog signalling. Cell Cycle 2008; 7:127 - 134
- Santos SD, Verveer PJ, Bastiaens PI. Growth factor- induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat Cell Biol 2007; 9:324 - 330
- Tian T, Harding A, Inder K, Plowman S, Parton RG, Hancock JF. Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat Cell Biol 2007; 9:905 - 914
- Das J, Ho M, Zikherman J, Govern C, Yang M, Weiss A, et al. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell 2009; 136:337 - 351
- Petersen CC, Malenka RC, Nicoll RA, Hopfield JJ. All-or-none potentiation at CA3-CA1 synapses. Proc Natl Acad Sci USA 1998; 95:4732 - 4737
- Sweatt JD. Toward a molecular explanation for long-term potentiation. Learn Mem 1999; 6:399 - 416
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci 2002; 5:295 - 296
- Fukunaga K, Stoppini L, Miyamoto E, Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 1993; 268:7863 - 7867
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of longterm potentiation. Proc Natl Acad Sci USA 1993; 90:8342 - 8346
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 2001; 31:191 - 201
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 2002; 3:175 - 190
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, et al. PKMzeta maintains spatial, instrumental and classically conditioned long-term memories. PLoS Biol 2008; 6:2698 - 2706
- Softky WR. Simple codes versus efficient codes. Curr Opin Neurobiol 1995; 5:239 - 247
- Marchetti C. Spike trains and kinase II for a digital model of long term memory (An exercise in evolutionary constraints). Human Evolution 2000; 15:187 - 197