Abstract
The bacterial pathogen Agrobacterium tumefaciens produces the quorum-sensing (QS) signal 3-oxo-octanoylhomoserine lactone (OC8HSL) for controlling horizontal transfer of its tumor inducing (Ti) plasmid that carries both the T-DNA and the virulence genes. Over-accumulation of OC8HSL also increases severity of plant symptoms (number of emerging tumors at infection site) by an unknown mechanism. A. tumefaciens strain C58 expresses two lactonases, AttM (BlcC) and AiiB, that cleave OC8HSL and are potential modulators of QS. Recent data highlight the direct contribution of lactonases AttM and AiiB in the control of OC8HSL level and QS-regulated functions such as conjugation of Ti plasmid and seriousness of plant symptoms. Expression of the two lactonases is regulated by different plant signals. A working model of QS in the course of the A. tumefaciens-plant host interaction is proposed and discussed.
Introduction
The bacterial phytopathogen Agrobacterium tumefaciens,Citation1 causative agent of the crown gall disease, is able to transfer a segment of DNA (T-DNA) from its tumor inducing (Ti) plasmid to the nuclear genome of the infected plant cells. T-DNA genes encode for synthesis of plant growth factors (auxin and cytokinins) resulting in uncontrolled cell proliferation and development of a plant tumor, in which intercellular spaces are colonized by the pathogen. In transformed plant cells, T-DNA genes also encode for synthesis of specific compounds, called opines, which are used by the pathogen as a source of nitrogen and carbon. In addition, some opines stimulate the synthesis of a bacterial quorum-sensing (QS) signal, 3-oxo-octanoylhomoserine lactone (OC8HSL), which in turn controls amplification of the copy number of the Ti plasmid in A. tumefaciens, and its horizontal transfer by conjugation. Citation2 QS is therefore a key regulatory element in the dissemination process of virulence genes. Moreover, the link between a high concentration of QS signal and aggravated virulence symptoms on plant hosts was established.Citation3–Citation5 This correlation suggests that QS contributes to A. tumefaciens aggressiveness. In this review, we discuss recent advancesCitation6–Citation8 relative to the implication of the lactonases AttM and AiiB in cleavage of the QS signal, therefore in modulation of the QS-mediated communication in A. tumefaciens.
The Lactonases AttM and AiiB
In A. tumefaciens strain C58, two lactonase-encoding genes, attM (=blcC=atu5139) and aiiB (=atu6071) are borne on At and Ti plasmids, respectively.Citation9,Citation10 The eponym proteins AttM and AiiB belong to two sub-clusters of a large family of Zn-hydrolasesCitation11 that encompasses QS signal-cleaving lactonases of Arthrobacter, Bacillus, Klebsiella, Mezorhizobium, Photorhabdus and Rhizobium. Both AttM and AiiB cleave the gamma-butyrolactone ring of a wide spectrum of QS signals.Citation9,Citation10 The structure of AiiB is availableCitation12 and shows high similarity with that of the QS-signal lactonase AiiA from Bacillus thuringiensis. Purified AiiB preferentially cleaves QS signals with an acyl chain longer than four carbons, such as hexanoyl-, octanoyl- and decanoylhomoserine lactones. AttM, but not AiiB, efficiently cleaves the simplest gamma-butyrolactone (GBL) into gamma-hydroxybutyric acid (GHB) that may be used as a carbon source by A. tumefaciens.Citation13,Citation14 Noticeably, A. tumefaciens cannot use QS signals as a sole carbon or nitrogen source.
Expression of attM and aiiB Genes is Controlled by Different Plant Signals
None of the tested QS-signals, including the A. tumefaciens-produced OC8HSL, were able to regulate the expression of the aiiB and attM genes.Citation7,Citation9,Citation13,Citation14 Transcription of these genes is, however, stimulated by different plant compounds. Expression of aiiB is increasedCitation7 in the presence of specific opines, agrocinopines A and B,Citation15 which are also required for synthesis of the QS signal OC8HSL in A. tumefaciens strain C58.Citation16,Citation17 Expression of attM gene, which belongs to the attKLM operon,Citation9 is increased in the presence of succinic semialdehyde (SSA), gamma-hydroxybutyrate (GHB), gamma-butyrolactone (GBL), gamma-aminobutyrate (GABA), and salicylic acid.Citation4,Citation13,Citation14,Citation18–Citation20 GABA acts as an ubiquitous signal implicated in communication between cells or organisms, including plants and bacteria.Citation21 AttM-inducing compounds, such as GABA and salicylic acid, are accumulated in A. tumefaciensinfected plants and A. tumefaciens-induced plant tumors.Citation22,Citation23 The plant pathways for GABA biosynthesis and degradation are strongly activated in A. tumefaciens-induced tumors;Citation22 and byproducts of GABA, such as SSA, GHB and GBL are therefore expected to be present in the plant tumors.Citation24
In vitro, SSA and GHB, but neither GBL nor GABA, are able to inhibit the DNA-binding capability of the transcriptional repressor AttJ at the promoter region of the attKLM operon.Citation14,Citation18 In cell cultures, the expression of attKLM observed in the presence of GABA and GBL should therefore require the conversion of these two compounds to SSA and GHB by a GABA-transaminase (an unidentified enzyme) and lactonase (AttM), respectively.Citation8 Under starvation, the stress alarmone ppGpp would stimulates biosynthesis of SSA, hence expression of lactonase AttM and decay of QS signal.Citation18 Because salicylic acid affects growth rate of A. tumefaciens,Citation19 one could hypothesize that, under salicylic acid-induced slowdown, A. tumefaciens could accumulate SSA which in turn stimulates AttM-mediated inactivation of the QS signal OC8HSL.
The mechanisms by which SSA, GHB and GBL penetrate A. tumefaciens cells are still unknown. However, both the ABC-transporter Bra (Atu2424 to Atu2427)Citation4 and periplasmic binding protein (PBP) Atu2422,Citation6,Citation25 are required for GABA uptake to the bacterial cytoplasm, and therefore for the GABA-induced expression of attKLM. There are more than 150 ABC transporters and PBPs in the genome of A. tumefaciens C58,Citation26 which are implicated in key functions including the binding of plant sugars by the PBP ChvE during the early steps of activation of the virulence genes,Citation27 and importation of opines, such as nopaline and agrocinopines. Citation28,Citation29 Noticeably, the agrocinopine ABC-transporter is required for expression of the lactonase AiiB.Citation7
A recent workCitation6 revealed that free amino acids such as proline (Pro) and valine may antagonize the uptake of GABA, therefore the GABA-induced degradation of OC8HSL. Because Pro accumulates in plant tumor,Citation22,Citation30 it may be a very probable natural antagonist of GABA signaling in A. tumefaciens. The mechanism by which Pro blocks importation of GABA implicates the PBP Atu2422 which is required for transport of Pro and GABA. Pro would act as a competitive antagonist of GABA for binding Atu2422. Remarkably, Pro does not affect the importation of GHB, suggesting that by-products of GABA may induce expression of attM gene even in the presence of Pro. A model of the complex regulation of AttM-encoding gene by plant compounds is proposed in the .
The Lactonases AttM and AiiB Modulate QS and Conjugation of Ti Plasmid
In A. tumefaciens strain C58, conjugation of Ti plasmid requires the presence of particular opines called agrocinopines and that of the QS-signal OC8HSL.Citation16,Citation17 Agrocinopines, which are imported into bacterial cytoplasm via the ABC transporter Acc,Citation28 modulate the repressor activity of the transcriptional factor AccRCitation31 that controls the expression of the operon encoding TraR. TraR exhibits a high affinity to OC8HSL. The TraR-OC8HSL complexCitation32,Citation33 binds specific DNA sequences of promoting regions of the rep and tra operons and activates their transcription. The rep and tra genes control amplification of copy number and horizontal transfer of the Ti plasmid. The TraR-OC8HSL complex also stimulates the transcription of traI,Citation17 that encodes for synthesis of OC8HSL, and therefore stimulates a positive loop that amplifies synthesis of the OC8SHL signal. A strong negative control on biosynthesis of OC8HSL is exerted by TraM that binds TraR and disrupts the TraR-DNA complex.Citation34 In the presence of agrocinopines, the synthesis of TraR at a high rate compensates the antagonist effect of TraM.
The implication of the AHL lactonases AttM and AiiB in the accumulation of OC8HSL and QS-regulated functions, especially conjugation of Ti plasmid was evaluated in different laboratories. In vitro, purified lactonase AttM inactivated more rapidly free OC8HSL than OC8HSL bound by TraR,Citation8 suggesting that the intracellular TraR-OC8HSL complex would protect the QS signal from lactonase cleavage. In a strain constitutively expressing TraR, the intracellular level of OC8HSL remained unaffected by AttM because of a protecting effect of TraR.Citation8 In contrast, when cell cultures of A. tumefaciens C58 were induced by attM-inducers, such as GABA, GHB, GBL and SSA, or aiiB-inducers agrocinopines, the lactonases AttM and AiiB modulated the level of the extracellular OC8HSL.Citation4,Citation7,Citation8,Citation13 These observations revealed the implication of lactonases in the control of OC8HSL exchange between cells, therefore the QS-mediated communication.
Conjugation of plasmid Ti was compared using donor strains in which lactonase-encoding genes were mutated or not. These experiments were performed in planta and in minimal medium in the presence of agrocinopines or in traM and/or accR genetic backgrounds. An aiiB mutant and its corresponding wild type strain were used as donors in conjugation experiments: Ti plasmid transconjugants emerged earlier in crosses involving the lactonase AiiB-defective strain. Moreover, the higher conjugation efficiency of aiiB mutant was correlated both with an early appearance and a higher level of OC8HSL in culture medium, confirming the implication of AiiB in QS and Ti plasmid conjugation. Citation7 Regarding AttM, the other lactonase, an attM mutant and an attJ mutant (this latter constitutively express the AttM lactonase) were used as donors in conjugation experiments. The attJ mutant was affected for transfer of the Ti plasmid in minimal medium and in planta.Citation8,Citation9 However, the attM mutant was slightly more efficient a donor than its wild type counterpart for conjugation of Ti plasmid in planta.Citation8 More precisely, transconjugants appeared earlier with attM mutant than with wild type as donors, suggesting that attM exerts a transitory effect on conjugation. Two main non-exclusive hypotheses may be proposed to explain this transitory effect: (1) the AttM-mediated inactivation of OC8HSL could be balanced by a stronger synthesis of OC8HSL and TraR in plant tumor; (2) the GABA-controlled expression of attKLM in planta could be attenuated by another plant signal such as free proline, which accumulates in the tumor tissues and antagonizes the transport of GABA into A. tumefaciens, therefore GABA-induced degradation of OC8HSL.Citation6
The Lactonases AttM and AiiB, QS and Plant Symptoms
The implication of QS in the modulation of virulence (number of emerging tumors) was initially reported in A. tumefaciens strain R10 overexpressing traR3 and then observed in other A. tumefaciens isolates.Citation4,Citation5 It was hypothesized that over-accumulation of QS signal could increase Ti plasmid copy number, thereby enhancing the virulence of Agrobacterium. Mutations affecting lactonase-mediated degradation of QS signals are therefore predicted to favor accumulation of QS signal, hence seriousness of plant symptoms. Virulence assays performed on tomato and tobacco plants with attM and aiiB mutants confirmed this hypothesis.Citation7 Moreover, the use of transgenic plants in which the ratio between GABA and its antagonist Pro were modified, supports the hypothesis of a link between GABA-induced expression of lactonase AttM and severity of plant symptoms. Indeed, plants with a high GABA content exhibited less severe symptoms than wild type plants,Citation4 while plants with a high Pro content exhibited more severe symptoms than wild type plants.Citation6 Further investigations are required for understanding the precise mechanism by which QS and plant factors would modulate emergence of plant tumors.
A Working Model for QS in A. tumefaciens-Plant Host Interaction
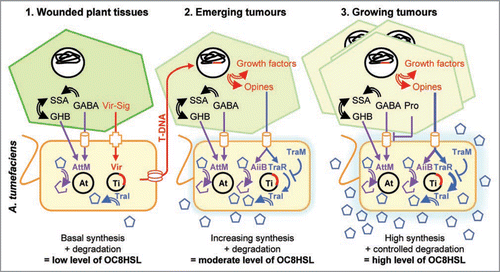
A working model for QS in A. tumefaciens-plant host interaction is shown on . In a first step, wounded plant released phenolic compounds (Vir-sig) that are sensed by the pathogen and stimulate the expression of the virulence genes. The vir genes encode the cellular machinery responsible for transfer of the T-DNA into the host cells and its integration in the plant nuclear genome. At the same time, the synthesis of the QS signal OC8HSL is low because of the absence of agrocinopines, while biodegradation of OC8HSL is potentially high because of the accumulation of GABA—and its by-products—in wounded plant tissues.
In a second step (emerging and young tumors), the expression of T-DNA genes in host plant cells drives biosynthesis of the plant growth factors, auxin and cytokinins, and that of opines, agrocinopines A and B and nopaline. While growth factors stimulate plant cell proliferation, opines are used by the pathogen as nutrients and signals for triggering biosynthesis of the OC8HSL-binding protein TraR. The complex OC8HSL-TraR positively controls biosynthesis of the OC8HSL at transcriptional level, but its DNA-binding activity is antagonized by the protein TraM that delays the work of a positive loop for OC8HSL biosynthesis and expression of the rep and tra operons. At the same time, agrocinopines and GABA—and its derivatives—may induce the expression of lactonase AiiB and AttM that cleave unbound OC8HSL, and therefore prevent accumulation and release of free OC8HSL in extracellular environment. The proposed second step would be the more impacted by lactonases because of a low synthesis rate of OC8HSL and TraR. In lactonase-defective strains, OC8HSL would accumulate earlier than in a wild type strain, and promote horizontal transfer of Ti plasmid and emergence of plant symptoms.
In a third step (growing tumors), an elevated production of agrocinopines by the transformed plant cells induces a strong biosynthesis of TraR that overcomes the antagonist TraM. TraR-OC8HSL complex stimulates biosynthesis of OC8HSL at a high rate that may also overcome the lactonase-mediated degradation of OC8HSL. At the same time, the accumulated free proline in plant tumor would antagonize the GABA-controlled expression of the lactonase AttM, therefore would decrease its OC8HSL-degradation capability. Indeed, OC8HSL signals are abundantly released in bacterial cell environment. The proposed working model should be tested by further in vivo experiments.
As a conclusion, A. tumefaciens C58 exhibits a fine control of biosynthesis and biodegradation of QS signal OC8HSL of which the ecological and evolutionary significance have to be explored. Moreover, the A. tumefaciens system may help us to understand the dynamics of biosynthesis and degradation of QS signals in the soil bacterial communities,Citation35 as well as their engineering for protecting plants.Citation36
Abbreviations
| ABC | = | ATP-binding cassette |
| GABA | = | gamma-aminobutyric acid |
| OC8HSL | = | 3-oxo-octoanoylhomoserine lactone |
| QS | = | quorum-sensing |
| T-DNA | = | transferred DNA |
| Ti | = | tumor inducing |
Figures and Tables
Figure 1 Regulation of AttM-encoding gene by plant signals. The upper part summarizes knowledge on the catabolic and QS-silencing functions of the attKLM operon, its regulation by transcriptional repressor AttJ, as well as the antagonistic activity of Pro for importation of GABA in the bacterial pathogen. The lower part illustrates the synthesis and degradation of GABA in plants. In addition to abbreviations used in the text: αKG, alpha-ketoglutarate; GAD, glutamate decarboxylase; GABA-T, GABA transaminase; GLYR, Glycolate reductase; SSADH, SSA dehydrogenase; Suc, succinate; TCA, tricarboxylic acid cycle. Simple and stopped black arrows represent regulatory pathways in A. tumefaciens; blue arrows, movements across cell compartments; double-line arrows, enzymatic reactions; and cylinder indicates the bacterial ABC-transporter Bra.
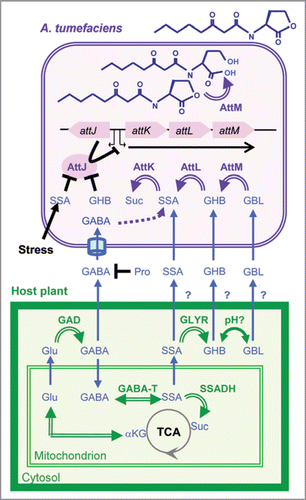
Figure 2 A working model for QS regulation in A. tumefaciens C58-plant host interaction. See text for details. Simple and stopped arrows represent regulatory pathways, whereas double-line arrows, cylinders, cross, and pentagons represent enzymatic reactions, bacterial ABC-transporters, VirA/G system, and OC8HSL, respectively.
Acknowledgements
E.H. and D.F. thank Y. Dessaux (CNRS, Gif-sur-Yvette) for a critical review of the manuscript. E.H. was funded by Ecole Doctorale Science du Végétal (ED145); D.F. by CNRS.
References
- Escobar MA, Dandekar AM. Agrobacterium tumefaciens as an agent of disease. Trends Plant Sci 2003; 8:380 - 386
- White CE, Winans SK. Cell-cell communication in the plant pathogen Agrobacterium tumefaciens. Philos Trans R Soc Lond B Biol Sci 2007; 362:1135 - 1148
- Pappas KM, Winans SC. A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes. Mol Microbiol 2003; 48:1059 - 1073
- Chevrot R, Rosen R, Haudecoeur E, Cirou A, Shelp BJ, Ron E, Faure D. GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci USA 2006; 103:7460 - 7464
- Molina L, Constantinescu F, Michel L, Reimmann C, Duffy B, Défago G. Degradation of pathogen quorum-sensing molecules by soil bacteria: a preventive and curative biological control mechanism. FEMS Microbiol Ecol 2003; 45:71 - 81
- Haudecoeur E, Planamente S, Cirou A, Tannières M, Shelp BJ, Moréra S, Faure D. Proline antagonizes GABA-induced quenching of quorum-sensing in Agrobacterium tumefaciens. Proc Natl Acad Sci USA 2009; 106:14587 - 14592
- Haudecoeur E, Tannières M, Cirou A, Raffoux A, Dessaux Y, Faure D. Different regulation and roles of lactonases AiiB and AttM in Agrobacterium tumefaciens C58. Mol Plant-Microbe Interact 2009; 22:529 - 537
- Khan SR, Farrand SK. The BlcC (AttM) Lactonase of Agrobacterium tumefaciens does not quench the quorum-sensing system that regulates Ti plasmid conjugative transfer. J Bacteriol 2009; 191:1320 - 1329
- Zhang HB, Wang LH, Zhang LH. Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc Natl Acad Sci USA 2002; 99:4638 - 4643
- Carlier A, Uroz S, Smadja B, Fray R, Latour X, Dessaux Y, Faure D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-Acyl homoserine lactonase activity. Appl Environ Microbiol 2003; 69:4989 - 4993
- Riaz K, Elmerich C, Moreira D, Raffoux A, Dessaux Y, Faure D. A metagenomic analysis of rhizospheric bacteria extends the diversity of quorum-quenching lactonases. Environ Microbiol 2008; 10:560 - 570
- Liu D, Thomas PW, Momb J, Hoang QQ, Petsko GA, Ringe D, Fast W. Structure and specificity of a quorum-quenching lactonase (AiiB) from Agrobacterium tumefaciens. Biochemistry 2007; 46:11789 - 11799
- Carlier A, Chevrot R, Dessaux Y, Faure D. The assimilation of γ-butyrolactone in Agrobacterium tumefaciens C58 interferes with the accumulation of the N-acyl-homoserine lactone signal. Mol Plant-Microbe Interact 2004; 17:951 - 957
- Chai Y, Tsai CC, Cho H, Winans SC. Reconstitution of the biochemical activities of the AttJ repressor and the AttK, AttL and AttM catbolic enzymes of Agrobacterium tumefaciens. J Bacteriol 2007; 189:3674 - 3679
- Ryder MH, Tate ME, Jones GP. Agrocinopine A, a tumor-inducing plasmid-coded enzyme product, is a phosphodiester of sucrose and L-arabinose. J Biol Chem 1984; 259:9704 - 9710
- Piper KR, Beck von Bodman S, Farrand SK. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 1993; 362:448 - 450
- Hwang I, Li PL, Zhang L, Piper KR, Cook DM, Tate ME, Farrand SK. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA 1994; 91:4639 - 4643
- Wang C, Zhang HB, Wang LH, Zhang LH. Succinic semialdehyde couples stress responses to quorum-sensing signal decay in Agrobacterium tumefaciens. Mol Microbiol 2006; 62:45 - 56
- Yuan ZC, Edlind MP, Liu P, Saenkham P, Banta LM, Wise AA, et al. The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc Natl Acad Sci USA 2007; 104:11790 - 11795
- Yuan ZC, Haudecoeur E, Faure D, Kerr KF, Nester EW. Comparative transcriptome analysis of Agrobacterium tumefaciens in response to plant signals salicylic acid, indole-3-acetic acid, and gammaamino butyric acid reveals signaling crosstalk and Agrobacterium-plant co-evolution. Cell Microbiol 2008; 10:2339 - 2354
- Shelp BJ, Bown AW, Faure D. Extracellular gamma-aminobutyrate mediates communication between plants and other organisms. Plant Physiol 2006; 142:1350 - 1352
- Deeken R, Engelmann JC, Efetova M, Czirjak T, Müller T, Kaiser WM, et al. An integrated view of gene expression and solute profiles of Arabidopsis tumors: a genome-wide approach. Plant Cell 2006; 18:3617 - 3634
- Lee CW, Efetova M, Engelmann JC, Kramell R, Wasternack C, Ludwig-Müller J, et al. Agrobacterium tumefaciens promotes tumor induction by modulating pathogen defense in Arabidopsis thaliana. Plant Cell 2009; 21:2948 - 2962
- Shelp BJ, Bown AW, McLean MD. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 1999; 4:446 - 452
- Moréra S, Gueguen-Chaignon V, Raffoux A, Faure D. Cloning, purification, crystallization and preliminary X-ray analysis of a bacterial GABA receptor with a Venus flytrap fold. Acta Crystallogr Sect F Struct Biol Cryst Commun 2008; 64:1153 - 1155
- Wood DW, Setubal JC, Kaul R, Monks DE, Kitajima JP, Okura VK, et al. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 2001; 294:2317 - 2323
- He F, Nair GR, Soto CS, Chang Y, Hsu L, Ronzone E, et al. Molecular basis of ChvE function in sugar binding, sugar utilization, and virulence in Agrobacterium tumefaciens. J Bacteriol 2009; 191:5802 - 5813
- Hayman GT, Beck von Bodman S, Kim H, Jiang P, Farrand SK. Genetic analysis of the agrocinopine catabolic region of Agrobacterium tumefaciens Ti plasmid pTiC58, which encodes genes required for opine and agrocin 84 transport. J Bacteriol 1993; 175:5575 - 5584
- Kim H, Farrand SK. Characterization of the acc operon from the nopaline-type Ti plasmid pTiC58, which encodes utilization of agrocinopines A and B and susceptibility to agrocin 84. J Bacteriol 1997; 179:7559 - 7572
- Wächter R, Langhans M, Aloni R, Götz S, Weilmünster A, Koops A, et al. Vascularization, high-volume solution flow, and localized roles for enzymes of sucrose metabolism during tumorigenesis by Agrobacterium tumefaciens. Plant Physiol 2003; 133:1024 - 1037
- Beck von Bodman S, Hayman GT, Farrand SK. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc Natl Acad Sci USA 1992; 89:643 - 647
- Qin Y, Luo ZQ, Smyth AJ, Gao P, Beck von Bodman S, Farrand SK. Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J 2000; 19:5212 - 5221
- Qin Y, Luo ZQ, Farrand SK. Domains formed within the N-terminal region of the quorum-sensing activator TraR are required for transcriptional activation and direct interaction with RpoA from Agrobacterium. J Biol Chem 2004; 279:40844 - 40851
- Qin Y, Su S, Farrand SK. Molecular basis of transcriptional antiactivation. TraM disrupts the Tra-RDNA complex through stepwise interactions. J Biol Chem 2007; 282:19979 - 19991
- d’Angelo-Picard C, Faure D, Penot I, Dessaux Y. Diversity of N-acyl homoserine lactone-producing and -degrading bacteria in soil and tobacco rhizosphere. Environ Microbiol 2005; 7:1796 - 1808
- Cirou A, Diallo S, Kurt C, Latour X, Faure D. Growth promotion of quorum-quenching bacteria in the rhizosphere of Solanum tuberosum. Environ Microbiol 2007; 9:1511 - 1522