Abstract
Wolbachia pipientis is known to infect only arthropods and nematodes (mainly filarial worms). A unique feature shared by the two Phyla is the ability to replace the exoskeleton, a process known as ecdysis. This shared characteristic is thought to reflect a common ancestry. Arthropod moulting is induced by the steroid hormone 20-hydroxyecdysone (20E) and a role for ecdysteroids in nematode ecdysis has also been suggested. Interestingly, Wolbachia removal in filarial worms is detrimental to the host that is subjected to an impaired development. From analyses of the genome of Wolbachia harboured by the filarial nematode Brugia malayi and that of its host, the bacterium may provide a source of heme, an essential component of cytochromes P450 that are necessary for biosynthetic pathways of steroid hormones.
In arthropods, Wolbachia is a reproductive manipulator, inducing various phenotypic effects that may be due to differences in host physiology, and in particular to endocrine-related processes governing development and reproduction. At this regard, insect steroids have a well-defined role in the coordination of multiple developmental processes, and in adults they control important aspects of reproduction, including ovarian development, oogenesis, sexual behaviour, and in some taxa vitellogenin biosynthesis.
According to some authors ecdysteroids may also act as sex hormones. In insect sex differentiation is generally thought to be a strictly genetic process, where each cell decides its own sexual fate based on its sex chromosome constitution, but, surprisingly, recent data demonstrate that in Drosophila sex determination is non cell-autonomous, as it happens in mammals. Thus the presence of signals coordinating the development of a gender-specific phenotype cannot be excluded. This could explain why Wolbachia interferes with insect reproduction; and also could explain why Wolbachia interferes with insect development. Thus, is “sex (= reproduction) and stripping (= ecdysis)” the key to the intimate relationship between Wolbachia and its host?
Introduction
Wolbachia is an intracellular alpha-proteobacterium, and is widespread in arthropods and in nematodes, mainly in filariae.Citation1 In both types of hosts, this bacterium is vertically transmitted from mother to offspring. Wolbachia is well known for its ability to manipulate the reproduction of arthropod hosts, while in filarial nematodes this bacterium is regarded as an obligate symbiont. Indeed, antibiotic treatments targeted toward Wolbachia impair the fertility of female adult filariae and block the development of the larvae, suggesting a role for Wolbachia in the oogenesis, embryogenesis and moulting of the host nematodes.Citation2–Citation4 Moulting, i.e., the ability to replace an exoskeleton, is a unique characteristic, shared by Arthropoda and Nematoda. High level phylogenies group these two phyla into the Ecdysozoa clade.Citation5 The fact that only arthropods and nematodes have thus far been found to harbour Wolbachia might thus reflect some hidden similarities in the intimate relationship between Wolbachia and its hosts.
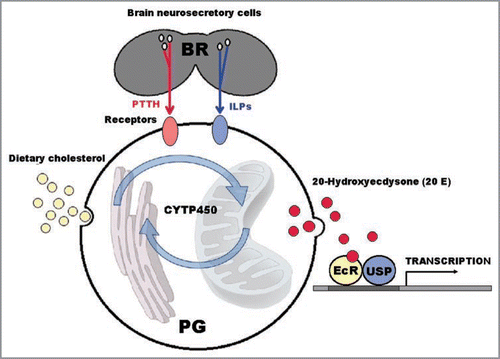
Insect moulting is induced by the steroid hormone 20-hydroxyecdysone (20E), whose precursor is secreted by prothoracic glands after their stimulation by the brain prothoracicotropic hormone (PTTH). Dietary cholesterol is converted to 20E thanks to hydroxylation reactions catalyzed by cytochrome P450 enzymes. 20E binds the heterodimeric nuclear receptor EcR/Usp (Ecdysone Receptor/Ultraspiracle), and activates the transcriptional processes underlying the cellular and morphogenetic moulting cascade events ().Citation6
In nematodes, as well, moulting seems to be regulated by ecdysteroid-like hormones and orthologs of insects nuclear receptors, involved in ecdysone response, have been found.Citation7,Citation8 In Caenorhabditis elegans these nuclear receptors are also involved in the regulation of sex determination and reproductive development.Citation9,Citation10
Interestingly, in filarial worms the removal of Wolbachia causes various detrimental effects on the host, which suffers impaired larval development.Citation11, Citation12 Combined analyses of the genomes of Wolbachia and its filarial host Brugia malayiCitation7,Citation13 suggest that the bacterium provides the nematode with heme, the prosthetic group of enzymes required for a variety of functions, including the biosynthesis of ecdysteroid hormones.Citation4,Citation14
In arthropods Wolbachia can induce cytoplasmic incompatibility, parthenogenesis, feminization, male-killing, fecundity enhancement, and even rescue of oogenesis defects. Such phenotypic variability is thought to be linked to high genome plasticity of insect-borne Wolbachia, since all of the genomes thus far sequenced for these symbionts contain high number of repetitive sequences, including IS (insertion sequences) elements and prophage-like sequences.Citation15
We argue that the various phenotypic effects observed in insects may be due to differences in host physiology, and in particular to endocrine-related processes governing development and reproduction which in insects display high variability.Citation16,Citation17 Insect steroids have a well-defined role in the coordination of multiple developmental processes, and in adults they control important aspects of reproduction, including ovarian development, oogenesis, and, in some taxa, vitellogenin biosynthesis.Citation16 In addition, recent data suggest a role for ecdysone and its receptors in sexual behavior.Citation18
According to De LoofCitation19 ecdysteroids may act as sex hormones. In insects sex differentiation is generally thought to be a strictly genetic process, in which each cell decides its own sexual fate based on its sex chromosome constitution,Citation20 but recent data demonstrate that, as in mammals, in the fruit fly Drosophila melanogaster non-autonomous sex determination controls sex dimorphism. Thus the presence of signals coordinating the development of a gender-specific phenotype cannot be excluded.
By carefully examining the available literature, in the following sections we propose a new perspective, supporting the role of Wolbachia in modulating the insect host sexual phenotypes by interaction with hormonal pathways, as it has already been shown for isopod crustaceans.Citation22
Feminization
Feminization is a phenomenon in which genetic male embryos carrying Wolbachia bacteria develop into females. In arthropods, feminization was first described in isopod crustaceans, including Armadillidium vulgare, Oniscus asellus and Porcellionides pruinosus.Citation23–Citation26 Later, feminization among insects was demonstrated in the Lepidopteran Eurema hecabeCitation27 and in the Hemipteran Zyginidia pullula.Citation28 In all these cases, the existence of intersexes has been described, and the feminizing effect was modulated by bacterial cell density.Citation29–Citation31
Authors generally state that in insects sex determination and differentiation are strictly genetic. This is supported by the observation that in Drosophila and the silkmoth Bombyx mori female specific genes are only expressed by female cells of gynandromorphs, aberrant specimens made up of both female cells (XX in Drosophila and ZW in B. mori, respectively) and male cells (XY in Drosophila and ZZ in B. mori, respectively).Citation32 How can one explain the existence of intersexes, i.e., individuals with an intermediate phenotype between male and female but genetically homogeneous pattern, if each cell makes its own sexual decision? If there were signals coordinating the development of a genderspecific phenotype, intersexes might arise from imbalance or conflict between male and female sex hormones and/or receptors. This has been confirmed by Rigaud and JuchaultCitation29 in the isopod A. vulgare, where intersexes are the result of a conflict between male differentiation and bacterial feminizing action.
In E. hecabe, Narita et al.Citation30 found that feminizing Wolbachia acts continuously throughout the larval development to produce the female phenotype. Since in lepidopteran sex determination is probably completed very early in embryogenesis, the authors speculate that the feminizing Wolbachia may interact with some female- or male-specific molecular mechanisms that are located downstream of the sex determination system and are responsible for the expression of female-specific phenotypes.Citation30 According to us, an interaction between the bacterium and the sex-differentiation process rather than the sex determination mechanism cannot be ruled out. If the bacteria does indeed act on sex differentiation, hormons are probably involved, and steroids play a key role during insect developmental processes. Some clues are provided by the work of Narita and colleaguesCitation30 itself. In infected butterflies, a partial Wolbachia curing during development, i.e., when host sex differentiation is not yet completed, leads to larva/pupal moulting defects. Some individuals do not pupate, while dissection of dead pupae reveals that many individuals failed to escape from the pupal case. These moulting defects resemble those observed in ecdysone receptor knock-out individuals of Blattella germanica and D. melanogaster EcR-mutants.Citation33,Citation34 Moreover, since in some Lepidopteran species ecdysteroid titre has been proven to regulate sex specific wing development,Citation35 sexually intermediate traits in wing morphology observed in E. hecabe specimens subjected to a partial Wolbachia curing could also be attributed to ecdysteroid action.Citation30
Clues of hypothetical interactions between W. pipientis and host hormone receptors are the following.
In Crustacea, which are phylogenetically close to insects, sexual differentiation and development of secondary sexual characteristics are under the control of sex hormones, as it is in vertebrates. Sex differentiation of Crustacea is driven by an androgenic hormone (AH), secreted by the androgenic gland (AG), whose action inhibits female differentiation.Citation22,Citation36,Citation37 Although no molecular data are available, the feminization effect induced by Wolbachia in crustaceans is thought to be linked to interactions between the bacterium and the AG differentiation process and/or AH receptors.Citation26,Citation29 Indeed, in genetic A. vulgare males, AH mRNA can be detected as soon as at the beginning of male gonad differentiation (Grève P, unpublished data). AH may thus have an early and local action by inducing male differentiation of embryonic gonads. Inherited Wolbachia could then induce feminization of genetic males by simply targeting AH receptor thereby inhibiting AG differentiation.Citation38 Wolbachia can also partially feminize adult males when experimentally inoculated by inducing female genital apertures and hypertrophy of AGs, presumably because AH receptors are not longer functional. This effect is similar of the one observed in male intersexes that have not fully transformed into females due to low bacterial load.Citation29
Interestingly, the AH was found to be a member of the evolutionarily related insulin and/or insulin-like growth factor families. This peptidic hormone consists of two chains linked by disulfide bridges, one of them glycosylated.Citation37,Citation39–Citation41 The mature hormone is derived from a precursor after excision of a C peptide, following maturation steps similar to the ones involved in insulin synthesis processes.Citation37,Citation43 These characteristics including proteolytic cleavage motifs and conserved cysteine residues seem to be a common feature of all isopod AHs studied so far (Grève P, unpublished data).
Studying the crayfish Cherax quadricarinatus, Manor et al.Citation42 point out that even if insulin and hormones members of the insulin family are generally not regarded as gender-specific, their expression in the crustacean AG is gender-specific, suggesting that they may have evolved in the context of regulating sexual differentiation. In fact, the crustacean AG regulates several sexrelated phenomena, such as male-like aggressive and reproductive behaviors, and even differential growth rate between male and female.Citation44,Citation45 In the shrimp Macrobrachium rosenbergii, in vivo silencing of the gene encoding an insulin-like AG peptide led to the arrest of testicular spermatogenesis accompanied by hypertrophy of the AGs and prevented the regeneration of male secondary sexual characteristics, but also induced a lag in molt and a reduction in growth parameters.Citation46
Insulin-like peptides (ILPs) are also found in insects. Bombyxins, a family of peptides produced by the brain of B. mori, were first of these found to have a structural homology to vertebrate insulins. Subsequent studies on Drosophila homologs revealed the existence of multigene families, expressed in insect brain and other tissues. The ILPs are among the major products synthesized by the median neurosecretory cells of the insects’ brain and may thus be considered as true neurohormones.Citation47,Citation48 ILPs regulate diverse functions, including growth, metabolism, fecundity and lifespan. Moreover some ILPs are predicted to be more similar to insulin-like growth factors (IGFs) than to insulin. They have distinct domain organizations and physiological functions, and they also differ in the mode of secretory regulation: for example, IGFs-like peptides are predominantly produced by the fat body, a functional equivalent of the vertebrate liver and adipocytes.Citation49 Interestingly, ILPs have an indirect effect on insects’ steroidogenesis through the stimulation of the gland growth.Citation50 In fact, two ecdysteroidogenetic axes are known in insects, the brain-prothoracic gland axis involved in development, and the brain-gonad axis involved in reproduction. In the first axis, ecdysteroid synthesis is triggered not only by protothoracicotropic hormone (PTTH), but also by insulin-like peptides (ILPs), secreted by insect brain.Citation47 In the second axis, ecdysteroid synthesis in the gonads (e.g., follicular cells in ovaries) is triggered by gonadotropins and, again, ILPs.Citation51
In Drosophila the chico gene encodes a major insulin receptor substrate involved in growth regulation.Citation52 Mutations in chico impair the proliferation of ovarian follicle cells and block egg chamber progression into vitellogenesis. Thus, the mutation blocks the insulin signalling needed for yolk protein synthesis and uptake in the ovary.Citation53 Homozygous chico females are sterile, but in presence of Wolbachia these mutant lines produce progeny.Citation54 Thus, Wolbachia action on hormonal pathways involving ecdysteroidogenesis could circumvent this insulin pathway defect (i.e., sterility).
Moreover, a recent study on Drosophila mutants for insulin/IGF-like signaling (IIS) (individuals characterized by moderate dwarfism, reduced fecundity and extension of female lifespan) indicates that Wolbachia may increase insulin signaling. In fact, symbiont removal further reduces IIS, enhancing IIS-related phenotypes such as extreme dwarfism, sterility, increased fat levels and shortened lifespan.Citation55
Male-Killing
Among the reported cases of male-killing Wolbachia, those of Ostrinia scapulalis and O. furnacalis are particularly intriguing.Citation56,Citation57 In these two moths Wolbachia kills genetic males (ZZ) during the larval stage, while genetic females (WZ) do not survive in absence of the bacterium. As in the Eurema hecabe feminization case, incomplete curing of male-killing Wolbachia infection produces Ostrinia intersexes.Citation56,Citation57 The authors considered these individuals partly feminized males, whereas complete feminization seemed to be incompatible with the survival of the male genotype. This leads to the hypothesis that male-killing is just an unsuccessful “attempt” at feminization by Wolbachia. Is this another density-dependent effect induced by the bacterium on the host hormonal system during development and sex differentiation?
In all these cases, sex-specific killing action by Wolbachia occurs during host development; in others, male-killing occurs during embryogenesis. It should be noted that embryogenesis takes place in a steroid hormone-enriched environment, and steroid hormones act for the coordination of morphogenetic movements.Citation58,Citation59
In Drosophila ecdysteroid levels begin to rise during early embryogenesis around the time of gastrulation and peak at stage 11–12 during germ band retraction (GBR), which is one of the major morphogenetic movements shaping the body plan of the first instar larva.Citation58 This happens about the time when the expression of genes shadow (sad) and disembodied (dib), coding for key enzymes of ecdysone biosynthesis (i.e., P450 cytochromes), show a striped pattern.Citation60
The only observations available on male embryonic death mechanisms involve Drosophila and the male-killer bacterium Spiroplasma: male embryonic development arrests before segmentation, just around the point of GBR.Citation61 Thus, if male-killing Wolbachia interacts with the ecdysteroidogenic pathways, this could interfere with the spatial and temporal expression patterns of cytochrome P450s required for a normal development of males.
Unfortunately, little information about sex-specific action of ecdysteroids during embryogenesis and development in insects is available, and it mainly concerns the effects of endocrinedisrupting chemicals. However, in vertebrates several studies on endocrine disruption indicate that some chemicals may have sex-specific effects, effects on sex differentiation and alterations in the sex ratio.Citation62 Indeed, in the housefly Musca domestica the sex ratio was one of several phenotypes to be affected by bisphenol A, a chemical that interferes with ecdysteroid-dependent physiological processes.Citation63 In particular, the sex-ratio shifted toward males when eggs and larvae were exposed to the chemical treatment.Citation63 Similar results were obtained on larvae of the moth Platynota idaeusalis after treatment with the molting-hormone agonist tebufenozide,Citation64 but tebufenozide treatment on larvae of the midge Chironomus riparius produced a femail-biased sex ratio.Citation65 According to the authors, the observed sex-specific effect could be explained by considering insect steroids sex hormones, with 20-hydroxyecdysone (20E) as the insect equivalent of vertebrate estrogen and its precursor ecdysone (E) as the insect equivalent of vertebrate testosterone. According to this view, in C. riparius tebufenozide might simulate higher 20E (i.e., female hormone) and consequently lower E levels (i.e., male hormone), when 20E binds to the ecdysone receptors. The likely induction of enzymes that metabolize E into 20E may lead to a further decrease in the E titer. This combination might affect male pupae more than female, so that male pupae die because they are subjected to an unsuitable, i.e., female, hormonal environment.Citation65
Conclusion
An interaction between Wolbachia and host hormonal pathways involving ecdysteroids may suggest the mechanistic way the bacterium uses for manipulating the host’s sexual behavior, opening new work hypothesis for the study of the evolutionary interactions between bacteria and host.
In all the known Wolbachia/host systems, the mechanisms underlying the interaction should be the same and the various phenotypic effects observed may be due to differences in host physiology, considering that endocrine-related processes governing host development and reproduction display an enormous variability.
We may also speculate that the various phenotypic effects induced by Wolbachia reflect an evolution underway between the symbiont and its host. In particular, effects on the host may range from negative to positive, as Wolbachia may be a reproductive manipulator, a facultative or an obligate symbiont, where it becomes essential for host survival or host fertility.Citation66–Citation68
Theory predicts that maternally-transmitted endosymbionts will be selected towards mutualism, increasing the fecundity of their female hosts. Detrimental effect on its host should result in selection for the bacteria to evolve a more benign lifestyle, changing the bacterium from being parasitic to more mutualistic.Citation67,Citation68 Shifts from reproductive manipulator to beneficial symbiont, i.e., from an “imperfect feeling” between Wolbachia and its host to a more perfect one, are documented and can also be rapid.Citation68
A further clue of this dynamic association might be the substantial variation in Wolbachia density among individuals of the same population, as observed in feminization, male-killing and cytoplasmic incompatibility, where the induced phenotype is positively correlated with the bacterium density.Citation31,Citation69
Thus, is sex and stripping the key to the (mostly) “unsteady” intimate relationship between Wolbachia and its host?
Figures and Tables
Figure 1 Insect moulting is induced by the circulating steroid hormone 20-hydroxyecdysone (20E) whose biosynthesis takes origin from dietary cholesterol. The process is initiated by secretion of the brain neuropeptide prothoracicotropic hormone (PTTH). PTTH acts on the prothoracic glands, triggering the synthesis and secretion of a pre-ecdysteroid which is sequentially converted to active 20E. Cholesterol is converted to 20E thanks to a number of hydroxylation reactions catalysed by cytochrome P450 enzymes of microsomal and/or mitochondrial origin. Then, 20E binds heterodimeric nuclear receptors composed of EcR (Ecdysone Receptor) and Usp (Ultraspiracle), and activates the transcriptional processes underlying the cellular and morphogenetic moulting cascade events. Even if PTTH is the the major tropic hormone driving ecdysone secretion, also insulin-like peptides (ILPs) may induce ecdysteroidogenesis, through stimulation of the prothoracic gland growth. BR (brain); ILPs (insulin-like peptides); PTTH (prothoracicotropic hormone); PG (prothoracic gland); EcR/USP (Ecdysone Receptor/Ultraspiracle).
References
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 2008; 6:741 - 751
- Bandi C, McCall JW, Genchi C, Corona S, Venco L, Sacchi L. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int J Parasitol 1999; 29:357 - 364
- Casiraghi M, McCall JW, Simoncini L, Kramer LH, Sacchi L, Genchi C, et al. Tetracycline treatment and sex-ratio distortion: a role for Wolbachia in the moulting of filarial nematodes?. Int J Parasitol 2002; 32:1457 - 1468
- Arumugam S, Pfarr KM, Hoerauf A. Infection of the intermediate mite host with Wolbachia-depleted Litomosoides sigmodontis microfilariae: impaired L1 to L3 development and subsequent sex-ratio distortion in adult worms. Int J Parasitol 2008; 38:981 - 987
- Ewer J. How the ecdysozoan changed its coat. PLoS Biology 2005; 3:1696 - 1699
- Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol 2002; 47:883 - 916
- Ghedin E, et al. Draft genome of the filarial nematode parasite Brugia malayi. Science 2007; 317:1756 - 1760
- Crossgrove K, Maina CV, Robinson-Rechavi M, Lochner MC. Orthologues of the Drosophila melanogaster E75 molting control gene in the filarial parasites Brugia malayi and Dirofilaria immitis. Mol Biochem Parasitol 2008; 157:92 - 97
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li TT, Li Y, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 2006; 124:1209 - 1223
- Höss S, Weltje L. Endocrine disruption in nematodes: effects and mechanisms. Ecotoxicology 2007; 16:15 - 28
- Bandi C, Trees AJ, Brattig N. Wolbachia in filarial nematodes: evolutionary aspects and implications for the pathogenesis and treatment of filarial diseases. Vet Parasitol 2001; 98:215 - 238
- Taylor MJ, Bandi C, Hoerauf A. Wolbachia bacterial endosymbionts of filarial nematodes. Adv Parasitol 2005; 60:245 - 284
- Foster J, et al. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol 2005; 3:121
- Brownlie JC, O’Neill SL. Wolbachia genomes: insights into an intracellular lifestyle. Curr Biol 2005; 15:507 - 509
- Iturbe-Ormaetxe I, O’Neill S. Wolbachia-host interactions: connecting phenotype to genotype. Curr Opin Microbiol 2007; 10:1 - 4
- Swevers L, Raikhel AS, Sappington TW, Shirk P, Iatrou K. Gilbert LI, Iatrou K, Gill SS. Vitellogenesis and post-vitellogenic maturation of the insect ovarian follicle. Comprehensive Molecular Insect Science. Reproduction and Development 2005; Oxford, UK Elsevier Ltd 87 - 156
- Raikhel AS, Brown M, Belles X. Gilbert LI, Iatrou K, Gill SS. Hormonal control of reproductive processes. Comprehensive Molecular Insect Science. Endocrinology 2005; Oxford, UK Elsevier Ltd 433 - 491
- Ganter GK, Walton KL, Merriman JO, Salmon MV, Brooks KM, Maddula S, Kravitz EA. Increased malemale courtship in ecdysone receptor deficient adult flies. Behav Genet 2007; 37:507 - 512
- De Loof A. Ecdysteroids: the overlooked sex steroids of insect? Males: the black box. Insect Science 2006; 13:325 - 338
- Schütt C, Nöthiger R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development 2000; 127:667 - 677
- DeFalco T, Camara N, Le Bras S, Van Doren M. Nonautonomous sex determination controls sexually dimorphic development of the Drosophila gonad. Dev Cell 2008; 14:275 - 286
- Legrand JJ, Legrand-Hamelin E, Juchault P. Sex determination in Crustacea. Biol Rev 1987; 62:439 - 470
- Martin G, Juchault P, Legrand JJ. Mise en évidence d’un micro-organisme intracytoplasmique symbiote de l’oniscoïde Armadillidium vulgare Latreille dont la présence accompagne l’intersexualité ou la féminisation totale des males génétiques de la lignée thélygène. C R Acad Sc Paris 1973; 276:231
- Rigaud T, Moreau J, Juchault P. Wolbachia infection in the terrestrial isopod Oniscus asellus: sex ratio distortion and effect on fecundity. Heredity 1999; 83:469 - 475
- Marcadé I, Souty-Grosset C, Bouchon D, Rigaud T, Raimond R. Mitochondrial DNA variability and Wolbachia infection in two sibling woodlice species. Heredity 1999; 83:71 - 78
- Bouchon D, Cordaux R, Grève P. Bourtzis K, Miller TA. Feminizing Wolbachia and evolution of sex determination in isopods. Insect symbiosis 2008; Boca Raton FL. Taylor and Francis Group LLC 273 - 294
- Hiroki M, Kato Y, Kamito T, Miura K. Feminization of genetic males by a simbiotic bacterium in a butterfly, Eurema hecabe (Lepidoptera: Pieridae). Naturwissenschaften 2002; 89:167 - 170
- Negri I, Pellecchia M, Mazzoglio PJ, Patetta, Alma A. Feminizing Wolbachia in Zyginidia pullula (Insecta, Hemiptera), a leafhopper with an XX/X0 sex-determination system. Proc R Soc B 2006; 273:2409 - 2416
- Rigaud T, Juchault P. Sterile intersexuality in an isopod induced by the interaction between a bacterium (Wolbachia) and the environment. Can J Zool 1998; 76:493 - 499
- Narita S, Nomura M, Kageyama D. Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiol Ecol 2007; 61:235 - 245
- Negri I, Franchini A, Gonella E, Daffonchio D, Mazzoglio PJ, Mandrioli M, Alma A. Unravelling the Wolbachia evolutionary role: the reprogramming of the host genomic imprinting. Proc R Soc B 2009; 276:2485 - 2491
- Fujii T, Shimada T. Sex determination in the silkworm, Bombyx mori: a female determinant on the W chromosome and the sex-determining gene cascade. Semin Cell Dev Biol 2007; 18:379 - 388
- Cruz J, Mané Padròs D, Belleés X, Martìn D. Functions of the ecdysone receptor isoform-A in the hemimetabolous insect Blattella germanica revealed by systemic RNAi in vivo. D Dev Biol 2006; 297:158 - 171
- Davis MB, Carney GE, Robertson AE, Bender M. Phenotypic analysis of EcR-A mutants suggests that EcR isoforms have unique functions during Drosophila development. Dev Biol 2005; 282:385 - 396
- Lobbia S, Niitsu S, Fujiwara H. Female-specific wing degeneration caused by ecdysteroid in the Tussock Moth, Orgyia recens: hormonal and developmental regulation of sexual dimorphism. J Insect Sci 2003; 3:1 - 7
- Juchault P, Legrand JJ. Croisement des néo-males expérimentaux chez Armadillidium vulgare Latr. (Crustacé Isopode Oniscoïde). Mise en évidence d’une hétérogamétie femelle. C R Acad Sci Paris 1972; 274:1387 - 1389
- Martin G, Sorokine O, Moniatte M, Bulet P, Hetru C, Van Dorsselaer A. The structure of a glycosylated protein hormone responsible for sex determination in the isopod, Armadillidium vulgare. Eur J Biochem 1999; 262:727 - 736
- Juchault P, Legrand JJ. Mechanism of the refractory state of androgen hormone in Armadillidium vulgare Latr. (crustacean, isopod, oniscoid) harboring a feminizing bacteria. Gen Comp Endocrinol 1985; 60:463 - 467
- Ohira T, Hasegawa Y, Tominaga S, Okuno A, Nagasawa H. Molecular cloning and expression analysis of cDNAs encoding androgenic gland hormone precursors from two porcellionidae species, Porcellio scaber and P. dilatatus. Zoolog Sci 2003; 20:75 - 81
- Okuno A, Hasegawa Y, Ohira T, Katakura Y, Nagasawa H. Characterization and cDNA cloning of androgenic gland hormone of the terrestrial isopod Armadillidium vulgare. Biochem Biophys Res Commun 1999; 264:419 - 423
- Grève P, Braquart-Varnier C, Strub J-M, Félix C, Van Dorsselaer A, Martin G. The glycosylated androgenic hormone of the terrestrial isopod Porcellio scaber (Crustacea). Gen Comp Endocr 2004; 136:389 - 397
- Manor R, Weil S, Oren S, Glazer L, Aflalo ED, Ventura T, et al. Insulin and gender: an insulin-like gene expressed exclusively in the androgenic gland of the male crayfish. Gen Comp Endocrinol 2007; 150:326 - 336
- Okuno A, Hasegawa Y, Nagasawa H. Purification and proprieties of androgenic gland hormone from the terrestrial isopod Armadillidium vulgare. Zool Sci 1997; 14:837 - 842
- Barki A, Karplus I, Khalaila I, Manor R, Sagi A. Male-like behavioral patterns and physiological alterations induced by androgenic gland implantation in female crayfish. J Exp Biol 2003; 206:1791 - 1797
- Manor R, Aflalo ED, Segall C, Weil S, Azulay D, Ventura T, Sagi A. Androgenic gland implantation promotes growth and inhibits vitellogenesis in Cherax quadricarinatus females held in individual compartments. Invertebr Reprod Dev 2004; 45:151 - 159
- Ventura T, Manor R, Aflalo ED, Weil S, Raviv S, Glazer L, Sagi A. Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 2009; 150:1278 - 1286
- Nässel DR. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Progr Neurobiol 2002; 68:1 - 84
- Wu Q, Brown MR. Signaling and function on insuline-like peptides in insects. Annu Rev Entomol 2006; 51:1 - 24
- Okamoto N, Yamanaka N, Satake H, Saegusa H, Kataoka H, Mizoguchi A. An ecdysteroid-inducible insulin-like growth factor-like peptide regulates adult development of the silkmoth Bombyx mori. FEBS J 2009; 276:1221 - 1232
- Truman JW. Steroid hormone secretion in insects comes of age. Proc Natl Acad Sci USA 2006; 24:8909 - 8910
- De Loof A. Ecdysteroids, juvenile hormone and insect neuropeptides: recent successes and remaining major challenges. Gen Comp Endocrinol 2008; 155:3 - 13
- Böhni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 1999; 97:865 - 875
- Richard DS, Rybczynski R, Wilson TG, Wang Y, Wayne ML, Zhou Y, et al. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J Insect Physiol 2005; 51:455 - 464
- Clark ME, Anderson CL, Cande J, Karr TL. Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics 2005; 170:1667 - 1675
- Ikeya T, Broughton S, Alic N, Grandison R, Partridge L. The endosymbiont Wolbachia increases insulin/ IGF-like signalling in Drosophila. Proc Biol Sci 2009; 276:3799 - 3807
- Kageyama D, Traut W. Opposite sex-specific effects of Wolbachia and interference with the sex determination of its host Ostrinia scapulalis. Proc R Soc B 2003; 271:251 - 258
- Sakamoto H, Kageyama D, Hoshizaki S, Yshikawa Y. Sex specific death in the Asian corn borer moth (Ostrinia furnacalis) infected by Wolbachia occurs across larval development. Genome 2007; 50:645 - 652
- Kozlova T, Thummel CS. Essential roles for ecdysone signaling during Drosophila mid-embryonic development. Science 2003; 301:1911 - 1914
- Gaziova I, Bonnette PC, Henrich VC, Jindra M. Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development 2004; 131:2715 - 2725
- Warren JT, Petryk A, Marqués G, Jarcho M, Parvy J-P, Dauphin-Villemant C, et al. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci USA 2002; 17:11043 - 11048
- Bentley JK, Veneti Z, Heraty J, Hurst GDD. The pathology of embryo death caused by the male-killing Spiroplasma bacterium in Drosophila nebulosa. BMC Biol 2007; 15:5 - 9
- Ankley G, et al. Overview of a workshop on screening methods for detecting potential (anti-) estrogenic/androgenic chemicals in wildlife. Environ Toxicol Chem 1998; 17:68 - 87
- Izumi N, Yanagibori R, Shigeno S, Sajiki J. Effects of bisphenol A on the development, growth and sex ratio of the housefly Musca domestica. Environ Toxicol Chem 2008; 27:1343 - 1353
- Biddinger D, Hull L, Huang H, McPheron B, Loyer M. Sublethal effects of chronic exposure to tebufenozide on the development, survival and reproduction of the tufted apple bud moth (Lepidoptera: Tortricidae). J Econ Entomol 2006; 99:834 - 842
- Hahn T, Liess M, Schulz R. Effects of the hormone mimetic insecticide tebufenozide on Chironomus riparius larvae in two different exposure setups. Ecotox Environ Safe 2001; 49:171 - 178
- Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci USA 2001; 98:6247 - 6252
- Moran NA, McCutcheon JP, Nakabach A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 2008; 42:165 - 190
- Weeks AR, Turelli M, Harcombe WR 3rd, Reynolds KT, Hoffmann AA. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol 2007; 5:114
- Unckless RL, Boelio LM, Herren JK, Jaenike J. Wolbachia as populations within individual insects: causes and consequences of density variation in natural populations. Proc R Soc B 2009; 276:2805 - 2811