Abstract
A tight spatio-temporal coordination of the machineries controlling actin dynamics and membrane remodeling is crucial for a huge variety of cellular processes that shape cells into a multicellular organism. Dynamic membrane remodeling is achieved by a functional relationship between proteins that control plasma membrane curvature, membrane fission and nucleation of new actin filaments. The BAR/ F-BAR-domain-containing proteins are prime candidates to couple plasma membrane curvature and actin dynamics in different morphogenetic processes. Here, we discuss recent findings on the membrane-shaping proteins of the F-BAR domain subfamily and how they regulate morphogenetic processes in vivo.
Introduction
During the development of multicellular organisms, numerous processes require a highly dynamic and organized interplay between cells and their environment. Cell migration, axonal pathfinding or the broad tissue rearrangements occurring during gastrulation are examples for such dynamic morphogenetic events. A prerequisite for these processes is the generation of force that drives cellular and intracellular movement and allows a cell to deform its membrane. One of the systems most commonly employed to generate force is the actin cytoskeleton.Citation1–Citation3 The actin filament system also contributes to development by regulating endocytosis, a fundamental process that is essential to downregulate or attenuate extracellular signaling, to shape morphogen gradients and to orchestrate cell fate decisions within a tissue. During endocytosis, actin generates the force that (1) drives membrane invagination, (2) helps to constrict the neck of a nascent vesicle, (3) aids the subsequent scission of the vesicle and (4) propels the vesicle away from the plasma membrane.Citation4–Citation9 Hence, it is easily conceivable that a tight regulation of the actin cytoskeleton and its intimate connection to the cell membrane is of great importance for a huge variety of developmental processes.
Over the past years, the Bin/Amphiphysin/Rvs (BAR) proteins have emerged as prime candidates to couple actin dynamics and membrane trafficking. The first BAR domain proteins that were identified are the yeast protein Reduced viability upon starvation (Rvs)161 and human Amphiphysin 1 (Amph1). Amph1 was found to be enriched in the brain and to associate with synaptic vesicles, whereas Rvs161 was identified in a screen for mutants defective in endocytosis.Citation10 This suggested a role for these proteins in membrane internalization and trafficking. The first study that found a direct interaction between membranes and Amph1 revealed that the N-terminal part of the protein was necessary and sufficient to deform lipid droplets into narrow tubules in vitro.Citation11 This N-terminal part of Amph1 was later identified as a novel membrane interacting protein domain, called BAR-domain, which is now the defining feature of all BAR proteins.Citation11–Citation14 This membrane tubulation activity observed in vitro is also relevant for the in vivo function of BAR domain superfamily proteins, as overexpression of these proteins or their N-terminal part in living cells induces the formation of hollow membrane tubules that extend into the cytoplasm and eventually pinch off from the plasma membrane.Citation15–Citation18
Structural Insights into the Role of BAR Domain Superfamily Proteins
The crystal structures of fifteen BAR family members have now been solved (summerized by McMahon H, at http://www.barsuperfamily.org). These studies revealed that BAR domains are basically composed of three anti-parallel coiled-coil helices which allow BAR family proteins to homodimerize.Citation19,Citation20 The crystal struct ures also revealed that the BAR domain superfamily is composed of several subfamilies, that differ slightly in the threedimensional arrangement of these coiled-coil motifs, such as the “classical” BAR proteins, the N-BAR proteins (that contain an N-terminal amphipathic helix), the F-BAR proteins (Fes/CIP4 homology BAR) and the I-BARs (Inverse-BAR).Citation21–Citation25
BAR domain dimers form a crescent-shaped surface that is covered with positively charged residues. These allow them to directly interact with negatively charged membrane lipids, such as Phosphoinositides or Phosphatidylserine.Citation19–Citation21,Citation26 Their ability to tubulate membranes and the crescent-shaped structure of the BAR domain dimers lead to the idea that these domains are used to sense and induce membrane curvature. A major advance in understanding the mechanism by which BAR proteins deform membranes was made by solving the crystal structures of the human Formin binding protein 17 (FBP17) and the Cdc42-interacting protein 4 (CIP4), two members of the F-BAR protein family.Citation17,Citation19,Citation27,Citation28 Shimada et al. (2007) found that FBP17 and CIP4 F-BAR domain dimers have the ability to associate with each other by end-to-end and lateral interactions. They form long filaments by joining end-to-end that wrap around a curved membrane, building a helical coat on its surface that is stabilized by lateral interactions. By this mechanism they stabilize the membranes’ curved shape and force it into a tubule of a specific diameter.Citation19,Citation21
F-BAR domains differ from classical BAR or N-BAR domains in that their concave surface has a larger diameter. Consistent with this, F-BAR proteins were found to force membranes into tubules with a wider diameter than BAR proteins.Citation19,Citation21 This suggests a sequential engagement of F-BAR and BAR domains in endocytosis. F-BAR proteins might induce and stabilize the initial, rather broad invagination of the endocytic pit, whereafter BAR proteins might help to narrow the stalk between the membrane and the endocytic vesicle, which aids the subsequent scission of the vesicle (). Consistently, F-BAR and BAR proteins segregate from each other on membrane tubules, thereby forming functionally distinct microdomains.Citation21
F-BAR Proteins Interconnect WASP/WAVE, Dynamin and the Membrane
F-BAR proteins were formerly recognized as a group of proteins termed Pombe Cdc15 homology (PCH) proteins. The archetypal feature of these proteins is their Fer/CIP4 homology (FCH) domain, which was shown to constitute a functional unit with a neighboring coiled-coil region, together forming the F-BAR domain.Citation16,Citation18,Citation27 The C-terminal part of these proteins can include various combinations of SH3, SH2, tyrosine kinase and RhoGAP domains. Accordingly, the mammalian F-BAR proteins have been grouped into subfamilies, the best described of which are the FBP17/CIP4-related PCH proteins (FCRPs), the synaptic dynamin-associated proteins (Syndapins) and the srGAP subfamily.Citation22,Citation24,Citation29 FCRPs induce tubular membrane invaginations in vitro and in vivo and participate in Dynamin-mediated endocytosis.Citation17 Knock down of any FCRP in COS-7 cells leads to a decrease in EGF-receptor uptake.Citation18 None of them is essential for EGFR endocytosis, but simultaneous knock down of all three FRCPs induces a much stronger effect, arguing that these proteins have redundant functions.Citation18
A second important feature of many F-BAR proteins is the presence of a Src homology 3 (SH3) domain that binds not only Dynamin and but also members of the Wiskott Aldrich Syndrome protein (WASP) family, that share their ability to activate the Arp2/3 complex and hence induce the nucleation of new actin filaments. The ability of FBP17 to interact with Neural (N)-WASP is also shared by CIP4 and Toca-1, the third member of the FCRPs, and all three proteins can recruit N-WASP to membrane tubules in cultured cells.Citation18,Citation24,Citation30 Initially, Toca-1 was reported to be an essential factor for N-WASP activation in Xenopus oocyte cell extracts, as depletion of Toca-1 from these extracts inhibited N-WASP mediated actin nucleation even in the presence of GTPbound Cdc42 or PIP2.Citation30 In later studies, however, this model of N-Wasp activation proved to be incomplete. Takano et al. (2008) found that Toca-1- and FBP17-mediated N-WASP activation is strongly enhanced in the presence of liposomes of a specific diameter, even in the absence of Cdc42.Citation31 This suggests, that the activation of N-WASP by FCRPs rather depends on their ability to recruit N-WASP to curved membranes than on an intrinsic ability of the FCRPs to directly activate N-WASP.
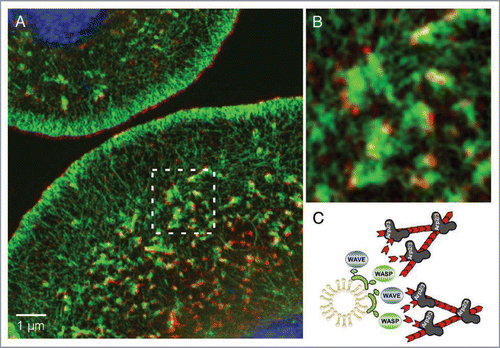
Recent studies in our group confirmed the idea that Cip4, the only FCRP in Drosophila, recruits WASP to promote Arp2/3-dependent vesicle movement in Drosophila S2R+ cells.Citation32 Structured-Illumination Microscopy (SIM) of Drosophila S2R+ cells stained for endogenous Cip4 protein and F-actin (phalloidin) documents Cip4-marked vesicles localizing at the tips of actin tails (). These findings are in good agreement with studies that showed that bacterial pathogens recruit human Toca-1 to their surface to induce N-Wasp dependent actin polymerization that drives their intracellular movement and cell-to-cell spread.Citation33 More importantly, we found that Drosophila Cip4 is able to form a complex not only with WASP but also with the related WASP family Verprolin homologous protein (WAVE, also called SCAR), and recruits both Nucleation promoting Factors (NPF) to invaginating membranes and endocytic vesicles. Actin comet tail-based movement of these vesicles depends not only on WASP but largely on WAVE function. Recently, evidence has accumulated that this previously unexpected interaction between FCRPs and WAVE proteins is well conserved throughout evolution. In C. elegans, Toca-1 and -2 bind to WAVE via Abi (as it is the case for Drosophila Cip4) and jointly regulate endocytosis in oocytes, while vertebrate Cip4 directly binds to Wave1 and regulates its phosphorylation by c-Abl.Citation34
These data lead to a model for FCRP function, in which these proteins first induce curvature of the plasma membrane and recruit further FCRP dimers that stabilize this initial invagination (). They form a helical coat around the endocytic pit that forces it to adopt a tubular shape with a specific diameter and enhances its further invagination (). (N-)BAR proteins are recruited to the membrane tubule and help to constrict its neck (). Hereafter, F-BAR and BAR proteins cooperatively recruit Dynamin to the neck, where it facilitates the scission of the vesicle (). Constriction and scission both are processes that also involve actin polymerization, indicating that WASP and WAVE proteins may also be recruited by FCRPs during these stages of endocytosis. Finally, F-BAR proteins remain associated with the vesicle, and recruit WASP and WAVE proteins that mediate the formation of actin comet tails that push the vesicle into the cell ().
The in vivo Function of BAR Proteins—Lessons from the Fly
Despite the detailed structural and biochemical knowledge about the mode of action of BAR proteins, our understanding how these proteins might function in a cellular and developmental context is still quite limited and complete loss-of-function situations are required to dissect the in vivo functions of BAR proteins. One of the very few BAR protein mutants that have been characterized in higher organisms is the mutant of the only Drosophila syndapin (synd) gene.Citation35 As mammalian Synd proteins are specifically enriched in the brain, their involvement in endocytosis and especially their interaction with Dynamin, lead to the idea that they are involved in synaptic vesicle endocytosis (SVE).Citation29,Citation36,Citation37 This idea is supported by the findings that inhibition of the Dynamin-Synd1 interaction and injection of a Synd1 antibody into axons both interferes with SVE in cultured neurons. Surprisingly, Kumar et al. (2009) found Drosophila Synd to localize postsynaptically at the larval neuromuscular junction (NMJ) and muscle-specific overexpression expands the postsynaptic membrane system, the subsynaptic reticulum (SSR,Citation35). In contrast, synaptic transmission and the rate of SVE are normal in synd null-mutants, suggesting that Syndapins might function differently in mammalian and invertebrate neurons.Citation38–Citation40 Also, Synd does not appear to be a general requirement for endocytosis or embryonic development, as maternal synd mutants die as pupae.Citation35 Similarly, the functional characterization of Drosophila amphiphysin (damph) null mutant have also failed to substantiate a role at synapses.Citation40–Citation42 Like Synd, Damph is enriched at the postsynapse and regulates the SNARE-dependent postsynaptic membrane cycling of the cell adhesion molecule Fasciclin II (FasII).Citation42
F-Bar proteins, however, are not dispensable for synaptic development and function in Drosophila, as mutations in nervous wreck (nwk) lead to an increase in bouton number, NMJ length and complexity as well as abnormal bouton morphology and impaired synaptic transmission.Citation43 nwk encodes for an F-BAR protein of the srGAP subfamily and contains a second SH3 domain instead of a RhoGAP domain. Nwk interacts with WASP and together they negatively regulate synaptic growth by mediating endocytosis of the bone morphogenic protein (BMP)—receptor Thickveins (Tkv) that relays positive growth signals from the muscle to the synapse.Citation44,Citation45
The genetic analysis of Drosophila cip4 mutants, the orthologue of the mammalian Cip4/Toca-1/FBP17 proteins, supports the idea that F-BAR proteins differentially regulate various processes.Citation32 The phenotypic analysis of cip4 mutant flies revealed a distinct requirement of Cip4 in controlling cell polarization in the developing wing.Citation32 In wild type wing epithelia, each wing cell forms a single hair, which is built on a scaffold of actin filaments. The site of actin polymerization is determined by sophisticated intercellular signaling of the Planar Cell Polarity (PCP-) pathwayCitation46,Citation47 although the underlying molecular mechanismen are still unclear. Loss of cip4 function results in the formation of multiple wing hairs. Interestingly, inhibition of Dynamin function also results in a wing hair duplication phenotype, an observation that highlights for the first time the importance of endocytic trafficking for wing hair formation. Further biochemical and genetic evidence strongly supports a model in which Cip4 forms distinct functional complexes with WASP and WAVE in a tissue-specific manner to couple endocytic processes to the actin machinery. Cip4 recruits WAVE to control Dynamin-dependent cell polarization in the wing whereas it acts through WASP to regulate the Dynamin-dependent endocytosis of E-cadherin in the dorsal thorax epithelium.Citation32,Citation48 Consistently, Giuliani and coworkers provided evidence for the requirement of Toca-1 and Toca-2 proteins in regulating distinct aspects of C. elegans morphogenesis through WASP and WAVE.Citation49
Like Synd, Nwk and Damph, Drosophila Cip4 is also not absolutely required for endocytosis as all of these mutants are viable.Citation43 Hence, different F-BAR proteins seemingly have evolved to facilitate the internalization of specific cell surface molecules by employing a common set of cellular factors in tissue-specific manner, rather than having a general function during endocytosis.
F-BAR Proteins are Implicated in the Pathogenesis of Human Diseases
So far, no vertebrate null-mutant for any FCRP has been described. Besides their well established role in endocytosis, some cell culture studies point to a role for FCRPs in regulating specific aspects of immune system function that have also been associated with WASP function. CIP4, for example, is required for the polarization of microtubules at the immunological synapse in natural killer cells, which is required for targeted secretion of lytic granules that act cytotoxic on infected cells.Citation50 FBP17, on the other hand, recruits WASP and Dynamin to podosomes and the phagocytic cup in macrophages,Citation51 which is required for the formation of both structures and an effective immune response.
Also, FCRPs have already been implicated in various human diseases. The gene encoding FBP17 has been shown to be fused to the mixed lineage leukemia (MLL) gene in some patients suffering from acute myelogenous leukemia.Citation52 Furthermore, a specific splice variant of CIP4 has been implicated in loss of beta-catenin-mediated cell adhesion in renal cancer and is associated with increased malignancy.Citation53 CIP4 and Syndapin both bind to Huntingtin, and both proteins are upregulated in brains of patients suffering from Huntington’s disease.Citation54,Citation55 Huntington’s disease (HD) is a dominant neurodegenerative disorder that is caused by polyglutamine (polyQ) expansion tract in Huntingtin, a ubiquitously expressed protein with unknown functions.Citation56 Numerous reports have described additional defects in the peripheral tissues of HD patients, including weight loss and altered glucose homeostasis. Mutant huntingtin impairs glucose-stimulated insulin secretion in insulin-producing beta-cells.Citation57 Interestingly, RNAi silencing of CIP4a resulted in increased insulin-stimulated glucose uptake in skeletal muscle cells, potentiated by an increase in surface GLUT4 glucose transporter. This suggests that Cip4 function also contributes to the pathogenesis of the HD diseaseCitation58 although the functional relationship between Cip4 and Huntingtin is still unclear. Compared to Cip4, the Wave-associated RacGAP protein (WRP), a members of the srGAP subfamily of F-BAR proteins, is functionally associated with the 3p syndrome.Citation59 Patients with 3p-syndrome who are haploinsufficient for WRP show severe mental retardation. WRP plays a critical role in the Slit-Robo signal-transduction pathwayCitation60 and directly binds WAVE1,Citation61,Citation62 suggesting that the pathogenesis is caused by a misregulation of the neuronal signal-transduction machinery controlling the correct migration of neurons and their axonal connectivity. However, the precise role of F-BAR proteins in the pathogenesis of these diseases is still unclear.
Outlook
Our understanding of the mechanisms how F-BAR proteins couple membrane dynamics and actin dynamics in vivo is still quite limited. One of the major tasks in the future will be to determine the correct time course and the mechanics of F-BAR protein-mediated endocytic processes. How does the helical F-BAR domain coat on membrane tubules interact with Dynamin, which also forms a helical coat on the vesicles neck to facilitate the subsequent scission of the vesicle? When exactly are actin nucleating proteins recruited to the membrane and for which steps of the process is their action required? It has become clear that both WASP and WAVE are employed by BAR/F-BAR proteins during endocytic processes, it is also an interesting question, whether (N-)WASP and WAVE act at the same stage during the endocytic process or whether they become differentially recruited at different steps. This picture might even become more complex, as novel members of the WASP protein family, such as WASH and WHAMM, have recently been identified, who also have a potential role in vesicle trafficking and might therefore also interact with F-BAR proteinsCitation63 and unpublished observations. Furthermore, studies in fission yeast and in vertebrate cell culture have revealed that some F-BAR proteins also interact with members of the Formin protein family of actin nucleators, an interaction, which also has not yet been in the focus of recent research.Citation64–Citation66
Although most F-BAR proteins appear to participate in membrane trafficking and endocytic events, some family members appear to have evolved to fulfill very different cellular functions. The srGAP protein srGAP2, for example, was recently shown to mediate the formation of membrane extensions rather than invaginations and is required for neuronal migration in the vertebrate brain.Citation67 Hence, it will be very interesting to see what other cellular and developmental processes members of this interesting protein family might regulate.
Figures and Tables
Figure 1 Model for BAR protein function during endocytosis. Schematic drawing, depicting the different steps at which F-BAR proteins are believed to function during endocyosis. (A) Induction of curvature: F-BAR protein dimers bind to and deform the plasma membrane. (B) Invagination/tubulation: By oligomerization, F-BAR proteins form a helical coat around the membrane, thereby stabilizing the invagination. They also might employ actin dynamics to generate the force for membrane invagination. (C) Constriction: (N-)BAR proteins associate with the neck of the tubule, mediating its constriction. (D) Dynamin recruitment: F- and (N-)BAR proteins recruit Dynamin, to also form a coat around the neck of the nascent vesicle, which will ultimately lead to the scission of the vesicle that is also aided by actin polymerization (E) Scission. (F) Vesicle movement: After the scission, WASP and WAVE proteins remain associated with the vesicle via F-BAR proteins, and mediate the formation of an actin tail that propels the vesicle into the cytoplasm.
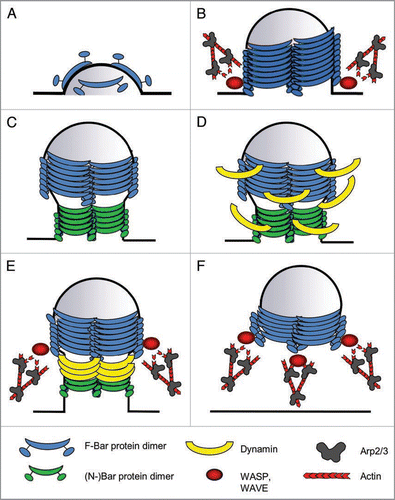
Figure 2 Cip4-marked vesicles localize at the tips of actin tails. (A) Structured Illumination Microscope (SIM) image of Drosophila S2 cells stained for endogenous Cip4 protein (red), F-actin (phalloidin, green). Nuclei were stained with DAPI (blue). (B) A magnified view of (A). Compared to other high-resolution microscopy techniques (e.g., STED) the SIM microscope achieves a resolution approaching 100 nm—double that of a conventional microscope without compromising on dyes or special sample treatment.Citation68
References
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003; 112:453 - 465
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct 2007; 36:451 - 477
- Insall RH, Machesky LM. Actin dynamics at the leading edge: from simple machinery to complex networks. Dev Cell 2009; 17:310 - 322
- Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell 2005; 16:964 - 975
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 2006; 7:404 - 414
- Takenawa T. From N-WASP to WAVE: key molecules for regulation of cortical actin organization. Novartis Found Symp 2005; 269:3 - 10
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 2007; 8:37 - 48
- Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol 2007; 19:453 - 458
- Disanza A, Frittoli E, Palamidessi A, Scita G. Endocytosis and spatial restriction of cell signaling. Mol Oncol 2009; 3:280 - 296
- Lichte B, Veh RW, Meyer HE, Kilimann MW. Amphiphysin, a novel protein associated with synaptic vesicles. EMBO J 1992; 11:2521 - 2530
- Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol 1999; 1:33 - 39
- Farsad K, et al. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol 2001; 155:193 - 200
- Balguerie A, Sivadon P, Bonneu M, Aigle M. Rvs167p, the budding yeast homolog of amphiphysin, colocalizes with actin patches. J Cell Sci 1999; 112:2529 - 2537
- Desfarges L, et al. Yeast mutants affected in viability upon starvation have a modified phospholipid composition. Yeast 1993; 9:267 - 277
- Lee E, et al. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 2002; 297:1193 - 1196
- Itoh T, et al. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell 2005; 9:791 - 804
- Kamioka Y, et al. A novel dynamin-associating molecule, formin-binding protein 17, induces tubular membrane invaginations and participates in endocytosis. J Biol Chem 2004; 279:40091 - 40099
- Tsujita K, et al. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol 2006; 172:269 - 279
- Shimada A, et al. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 2007; 129:761 - 772
- Peter BJ, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 2004; 303:495 - 499
- Frost A, et al. Structural basis of membrane invagination by F-BAR domains. Cell 2008; 132:807 - 817
- Aspenstrom P. Roles of F-BAR/PCH proteins in the regulation of membrane dynamics and actin reorganization. Int Rev Cell Mol Biol 2009; 272:1 - 31
- Chitu V, Stanley ER. Pombe Cdc15 homology (PCH) proteins: coordinators of membrane-cytoskeletal interactions. Trends Cell Biol 2007; 17:145 - 156
- Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta 2006; 1761:897 - 912
- Dawson JC, Legg JA, Machesky LM. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol 2006; 16:493 - 498
- Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell 2009; 137:191 - 196
- Aspenstrom P. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr Biol 1997; 7:479 - 487
- Chan DC, Bedford MT, Leder P. Formin binding proteins bear WWP/WW domains that bind proline-rich peptides and functionally resemble SH3 domains. EMBO J 1996; 15:1045 - 1054
- Kessels MM, Qualmann B. The syndapin protein family: linking membrane trafficking with the cytoskeleton. J Cell Sci 2004; 117:3077 - 3086
- Ho HY, et al. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell 2004; 118:203 - 216
- Takano K, Toyooka K, Suetsugu S. EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization. EMBO J 2008; 27:2817 - 2828
- Fricke R, et al. Drosophila Cip4/Toca-1 Integrates Membrane Trafficking and Actin Dynamics through WASP and SCAR/WAVE. Curr Biol 2009;
- Leung Y, Ally S, Goldberg MB. Bacterial actin assembly requires toca-1 to relieve N-wasp autoinhibition. Cell Host Microbe 2008; 3:39 - 47
- Roignot J, et al. CIP4 is a new ArgBP2 interacting protein that modulates the ArgBP2 mediated control of WAVE1 phosphorylation and cancer cell migration. Cancer Lett 2009;
- Kumar V, Alla SR, Krishnan KS, Ramaswami M. Syndapin is dispensable for synaptic vesicle endocytosis at the Drosophila larval neuromuscular junction. Mol Cell Neurosci 2009; 40:234 - 241
- Qualmann B, Kelly RB. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J Cell Biol 2000; 148:1047 - 1062
- Simpson F, et al. SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat Cell Biol 1999; 1:119 - 124
- Di Paolo G, et al. Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron 2002; 33:789 - 804
- Razzaq A, et al. Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev 2001; 15:2967 - 2979
- Zelhof AC, et al. Drosophila Amphiphysin is implicated in protein localization and membrane morphogenesis but not in synaptic vesicle endocytosis. Development 2001; 128:5005 - 5015
- Zhang B, Zelhof AC. Amphiphysins: raising the BAR for synaptic vesicle recycling and membrane dynamics. Bin-Amphiphysin-Rvsp. Traffic 2002; 3:452 - 460
- Mathew D, Popescu A, Budnik V. Drosophila amphiphysin functions during synaptic Fasciclin II membrane cycling. J Neurosci 2003; 23:10710 - 10716
- Coyle IP, et al. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron 2004; 41:521 - 534
- Rodal AA, Motola-Barnes RN, Littleton JT. Nervous wreck and Cdc42 cooperate to regulate endocytic actin assembly during synaptic growth. J Neurosci 2008; 28:8316 - 8325
- O’Connor-Giles KM, Ho LL, Ganetzky B. Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron 2008; 58:507 - 518
- Adler PN, Lee H. Frizzled signaling and cell-cell interactions in planar polarity. Curr Opin Cell Biol 2001; 13:635 - 640
- Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol 2008; 18:1555 - 1564
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol 2008; 18:1639 - 1648
- Giuliani C, et al. Requirements for F-BAR proteins TOCA-1 and TOCA-2 in actin dynamics and membrane trafficking during Caenorhabditis elegans oocyte growth and embryonic epidermal morphogenesis. PLoS Genet 2009; 5:1000675
- Banerjee PP, et al. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med 2007; 204:2305 - 2320
- Tsuboi S, et al. FBP17 Mediates a Common Molecular Step in the Formation of Podosomes and Phagocytic Cups in Macrophages. J Biol Chem 2009; 284:8548 - 8556
- Fuchs U, et al. The human formin-binding protein 17 (FBP17) interacts with sorting nexin, SNX2, and is an MLL-fusion partner in acute myelogeneous leukemia. Proc Natl Acad Sci USA 2001; 98:8756 - 8761
- Tsuji E, et al. Splicing variant of Cdc42 interacting protein-4 disrupts beta-catenin-mediated cellcell adhesion: expression and function in renal cell carcinoma. Biochem Biophys Res Commun 2006; 339:1083 - 1088
- Modregger J, DiProspero NA, Charles V, Tagle DA, Plomann M. PACSIN 1 interacts with huntingtin and is absent from synaptic varicosities in presymptomatic Huntington’s disease brains. Hum Mol Genet 2002; 11:2547 - 2558
- Holbert S, et al. Cdc42-interacting protein 4 binds to huntingtin: neuropathologic and biological evidence for a role in Huntington’s disease. Proc Natl Acad Sci USA 2003; 100:2712 - 2717
- DiFiglia M, et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron 1995; 14:1075 - 1081
- Smith R, et al. Mutant huntingtin interacts with {beta}-tubulin and disrupts vesicular transport and insulin secretion. Hum Mol Genet 2009; 18:3942 - 3954
- Hartig SM, et al. The F-BAR protein CIP4 promotes GLUT4 endocytosis through bidirectional interactions with N-WASp and Dynamin-2. J Cell Sci 2009; 122:2283 - 2291
- Endris V, et al. The novel Rho-GTPase activating gene MEGAP/srGAP3 has a putative role in severe mental retardation. Proc Natl Acad Sci USA 2002; 99:11754 - 11759
- Wong K, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell 2001; 107:209 - 221
- Soderling SH, et al. The WRP component of the WAVE-1 complex attenuates Rac-mediated signalling. Nat Cell Biol 2002; 4:970 - 975
- Soderling SH, et al. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity and memory. J Neurosci 2007; 27:355 - 365
- Campellone KG, Webb NJ, Znameroski EA, Welch MD. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell 2008; 134:148 - 161
- Carnahan RH, Gould KL. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J Cell Biol 2003; 162:851 - 862
- Wu JQ, et al. Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J Cell Biol 2006; 174:391 - 402
- Aspenstrom P. Formin-binding proteins: Modulators of formin-dependent actin polymerization. Biochim Biophys Acta 2009;
- Guerrier S, et al. The F-BAR domain of srGAP2 induces membrane protrusions required for neuronal migration and morphogenesis. Cell 2009; 138:990 - 1004
- Gustafsson MG, et al. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys J 2008; 94:4957 - 4970