Abstract
Majority of plant growth promoting rhizobacteria (PGPR) confer plant immunity against a wide range of foliar diseases by activating plant defences that reduce a plant’s susceptibility to pathogen attack. Here we show that Arabidopsis thaliana (Col-0) plants exposed to Bacillus subtilis strain FB17 (hereafter FB17), results in reduced disease severity against Pseudomonas syringae pv. tomato DC3000 (hereafter DC3000) compared to plants without FB17 treatment. Exogenous application of the B. subtilis derived elicitor, acetoin (3-hydroxy-2-butanone), was found to trigger induced systemic resistance (ISR) and protect plants against DC3000 pathogenesis. Moreover, B. subtilis acetoin biosynthetic mutants that emitted reduced levels of acetoin conferred reduced protection to A. thaliana against pathogen infection. Further analysis using FB17 and defense-compromised mutants of A. thaliana indicated that resistance to DC3000 occurs via NPR1 and requires salicylic acid (SA)/ethylene (ET) whereas jasmonic acid (JA) is not essential. This study provides new insight into the role of rhizo-bacterial volatile components as elicitors of defense responses in plants.
Introduction
Plants are constantly challenged by a plethora of disease causing microorganisms.Citation1,Citation2 To counter the onslaught of infections by microorganisms, plants have evolved a combination of constitutive and inducible defense responses.Citation3,Citation4 Induction of programmed cell death at the site of infection is a mechanism known as hypersensitive response (HR), while induction of defenses triggered throughout the plant is referred to as systemically induced resistance.Citation5 The mechanism of induction for HR is highly sophisticated and occurs as a result of gene for gene interactions between plant encoded resistance (R) genes and corresponding pathogen encoded avirulence (Avr) genes. The induction of SAR leads to enhanced resistance in distal parts of the plant against further attack by the same or a different pathogen.Citation6 This increase in systemic resistance occurs by two common pathways. These two pathways involve mediation by the plant hormones salicylic acid (SA) and JA. SA and JA defense responses are known to reduce the natural bacterial diversity on A. thaliana.Citation7,Citation8 Some of the SA analogs such as BTH (benzo(1,2,3) thiadiazole-7-carbothioic acid S-methyl ester) and INA (dichloroisonicotinic acid) also are known to elicit SAR.Citation9 Further SA is known to signal the expression of a number of pathogenesis related proteins (PRs) like PR-1, PR-2 and PR-5 in Arabidopsis thaliana and Nicotiana tabacum plants.Citation10 Studies involving genome wide transcript analysis of A. thaliana have revealed other genes, in addition to the known PRs likely to function in SAR.Citation11
A number of biocontrol bacteria, also known as plant growth promoting rhizobacteria (PGPR), protect plants from soil-borne pathogens by antagonistic mechanisms.Citation12,Citation13 Such bacteria colonizing on the roots can also induce systemic resistance in aerial plant parts, which are spatially separated from the inducing PGPR. The protection by such a mechanism typically is manifested by reduction in the pathogen growth and is phenotypically similar to the pathogen induced SAR.Citation14 This mechanism of induction of systemic resistance by root colonizing rhizobacteria in aerial plant parts is referred to as induced systemic resistance (ISR).
There are reports describing ISR elicitation by various Gramnegative Pseudomonas spp and Gram positive biocontrol PGPR Bacillus spp.Citation15–Citation21 A. thaliana plants treated with PGPR Serratia marcescens strain 90–166 and B. pumilis strain SE34 showed reduced disease severity and symptom development against Cucumber mosaic virus (CMV).Citation14 B. subtilis, found ubiquitously in the soil, has been reported to promote plant growth and protect plants against fungal infection,Citation14,Citation22–Citation27 apart from inducing ISR.Citation14 The induction of ISR when treated with PGPRs has been shown to be mediated primarily through plant signaling molecules such as JA and ethylene (ET).Citation14,Citation16
In addition to B. subtilis biocontrol against soil borne fungal pathogens, B. subtilis systemically protect A. thaliana against cucumber mosaic virus by SA and NPR1 independent and JA dependent mechanisms.Citation14 However, its biocontrol effect against A. thaliana root infection by P. syringae was attributed to its ability to form biofilms and to produce surfactin.Citation12 Few bacterial volatile components are reported to induce systemic resistance in plants against various pathogens,Citation28 although, it has been observed that bacterial volatile components (acetoin or 3-hydroxy-2-butanone) can serve as agents for triggering growth promotion in A. thaliana.Citation27 Additionally, it was also demonstrated that a volatile compound, 2,3-butanediol which is structurally similar to acetoin produced by Bacillus amyloliquefaciens IN937a and Bacillus subtilis GB03 elicited an ISR in Arabidopsis.Citation27 Plant components containing a six carbon backbone, e.g., (E)-2-hexenal, which is rapidly emitted from damaged plant tissue, has also been shown to induce the expression of defense-related genes in intact plants.Citation29,Citation30 In addition, a defensive role for terpenes as volatile elicitors has been proposed in excised lima bean (Phaseolus lunatus) plants.Citation29 A recent study, from our group, showed that DC3000 infected shoots relay chemical signal(s) underground through root secretions. The root-secreted chemical specifically attracts and enhances FB17 root binding and biofilm formation on the infected seedlings.Citation31 Although various groups have shown the mechanisms of B. subtilis biocontrol of root infections, the studies on the effect of B. subtilis induced ISR on aerial bacterial pathogenic infections are limited. Most of the reports relating to airborne signals inducing ISR are performed in isolation with respect to the biocontrol agent and the aerial pathogen. In this study, we report that root colonized B. subtilis (FB17) triggers acetoin induced ISR in the aerial parts. Further, ISR restricts pathogen multiplication and disease progression through a SA/ET and NPR1 dependent mechanism.
Results
B. subtilis root colonization reduces foliar disease.
To increase the understanding of the biological significance of FB17 root binding during aerial infection, we examined this symbiotic interaction in actual disease resistance. The FB17 root-colonized plant leaves, inoculated with DC3000, showed a reduced symptom development in terms of formation of water soaked lesions compared to controls with no FB17 root inoculation at 5 days post infection (). Further, a reduction in pathogen multiplication, as shown by a reduced number of colony forming units (CFU) counts of DC3000 in the inoculated leaves was observed in plants co-treated with FB17 (F(4,25) = 312.6, p < 0.05) compared to plants infected only with DC3000 ().
Acetoin, a volatile organic compound (VOC) produced by B. subtilis protects plants from DC3000 infection.
We examined whether the elicitation of defense pathways can be mediated by a bacterial volatile organic compound that protects plants from DC3000 infection. In this experiment, we included acetoin in the magenta boxes in which A. thaliana plants were grown. Interestingly, the plants subjected to volatile treatment developed much lower disease symptoms when compared to untreated controls (). Significantly lower DC3000 CFUs (p < 0.05, t-test) were recorded from the leaves of the plants subjected to acetoin treatment (). Since DC3000 was not susceptible to direct antimicrobial acetoin (1 ml of 10 mM stock ∼88 µg) treatments from broth micro-dilution assays (Suppl. Fig. 1), acetoin appears to be effectively activating plant defenses against aerially infected DC3000.
Acetoin biosynthetic mutants fail to trigger ISR.
To further probe the role of acetoin in induction of ISR in A. thaliana against DC3000 infections, we employed B. subtilis strains (BSIP1173 and BSIP1174) impaired in production of acetoin.Citation32 The genotypes of both acetoin nonproducing strains (BSIP1173 and BSIP1174) were trpC2 alsS::alsS-lacZ2 cat and trpC2 alsS::alsS-lacZ cat pta::aphA3 respectively.Citation32 The insertional inactivation of the als operon (BSIP1173) and double mutant (als and pta) (strain BSIP1174), lead to abolishment in acetoin production.Citation32 The mutant and overexpressing strain (BSIP1171) (genotype: trpC2 pta::aphA3) were tested and compared against wild type strains BS168 (parental strain), GB03 and FB17 for their effect on DC3000 infection in A. thaliana. All of the wild type and mutant strains, including the overexpressing strain BSIP1171, showed similar root colonization on aerially infected DC3000 A. thaliana Col-0 plants () but the acetoin mutant strains (BSIP1173 and BSIP1174) failed to protect the plants from DC3000 infection (). A significantly higher symptom development (), percent disease incidence (F(6,43) = 153.1, p < 0.05) () and DC3000 CFUs (F(6,43) = 125.3, p < 0.05) () were recorded from the plant roots that were inoculated with acetoin biosynthetic mutant strains compared to the plant roots inoculated with wild type B. subtilis strains (). In summary, our data shows that acetoin released from the B. subtilis augments plant defense against aerial DC3000 infections. We also show that acetoin is not important for the root binding phenotype as the acetoin mutant biofilms were indistinguishable from the parental wild type strainsCitation31,Citation33 ().
Interestingly, a precursor of acetoin, 2,3-butanediol did not protect plants against DC3000 infections. We used previously established in vitro infection protocol,Citation59 which relies on the degradation of chlorophyll florescence with the DC3000 inflicted disease progression as well as measuring bacterial growth through determining colony forming units per gram of fresh weigh of the seedlings. The A. thaliana plants treated with 2,3 butanediol and infected with DC3000 showed similar loss of red fluorescence (indicative of chlorophyll loss) compared to the lone DC3000 treatments (Suppl. Fig. 2). The results were interpreted using micrographs correlated well with the pathogen multiplication data set, wherein, plants treated with 2,3 butanediol and infected with DC3000 showed similar trends of pathogen multiplication compared to the lone DC3000 treatments (Suppl. Fig. 2). These results suggest the importance of acetoin but not its precursor 2,3 butanediol, in disease protection in A. thaliana against DC3000 pathogenesis.
FB17-inoculated A. thaliana mutants exhibit differential defense responses.
To determine the involvement of SA and ET/JA pathways mediated by FB17, we employed a SA deficient A. thaliana line (NahG) and ET/JA (etr1-3, jar1-1) A. thaliana signaling mutants treated with FB17 and infected with DC3000. Little difference was observed between the B. subtilis root inoculated and un-inoculated NahG plants in terms of disease symptom development (Suppl. Fig. 3), and DC3000 CFUs from inoculated leaves (). In contrast, a JA component mutant jar1-1 showed reduced DC3000 CFU (F(2,19) = 231.2, p < 0.05) and disease symptoms indicative of chlorosis post FB17 treatment (Suppl. Fig. 3; ). The ET pathway compromised mutant, etr1-3 revealed a similar phenotype as shown previously with NahG plants, indicating no enhanced disease resistance post FB17 treatment and DC3000 infection (Suppl. Fig. 3; ). Together, these data suggest that B. subtilis FB17 induced systemic resistance is NPR-1 dependent and requires SA and ET.
Further, to test whether acetoin also requires NPR1, SA and ET components to induce resistance against DC3000, infections were carried out a similar experiment as described earlier for FB17 except that the FB17 was replaced with acetoin treatment. The results presented in Supplementary Figure 3 show that acetoin failed to protect SA deficient NahG and ethylene mutant etr1-3 plants from DC3000 infection, whereas a significant reduction in DC3000 multiplication in terms of CFUs (F(2,29) = 231.2, p < 0.05) was observed with wild type Col-0 and JA mutant jar1-1 (). These results correlated well with the earlier results with FB17 treatment and indicated that the B. subtilis derived-acetoin functions through NPR1-dependent pathway and required SA and ET components to induce resistance against DC3000 infections.
B. subtilis and acetoin effect key genes in the SA and JA/ET pathways.
Our previous studies showed a higher PR1::GUS expression in plants that were root inoculated with FB17 compared to control untreated plants.Citation31 The PR1::GUS expression was higher in the aerial parts compared to the root system in the FB17 treated plants. A. thaliana leaves sprayed with SA also showed higher PR1 expression while the root treated plants showed much less PR1 expression.Citation31 As our results show that acetoin treated A. thaliana plants displayed enhanced resistance against DC3000, we examined whether acetoin induced expression of key defense genes. Further, PR1::GUS plants with acetoin showed enhanced PR1 expression compared to the untreated plants. Acetoin treated A. thaliana plants also revealed PR1 expression patterns similar to FB17 treatments (data not shown). Our data with the disease compromised mutants showed that FB17 mediated ISR, against DC3000, is NPR1-dependent and requires ET/SA. Further, we estimated the free SA levels, and our results showed significantly (F(8,55) = 175.2, p = 0.05) higher SA levels in the leaves of the plants that were root inoculated with FB17 compared to the control untreated plants (Suppl. Fig. 4A). Negative controls such as plant roots treated with other rhizobacteria P. aeruginosa (PAO1) and Pfo-1 showed no induction in free SA levels (Suppl. Fig. 4A). Interestingly, acetoin treatment also enhances the total free SA level (F(2,19) = 131.2, p < 0.05) in A. thaliana plants (Suppl. Fig. 4B).
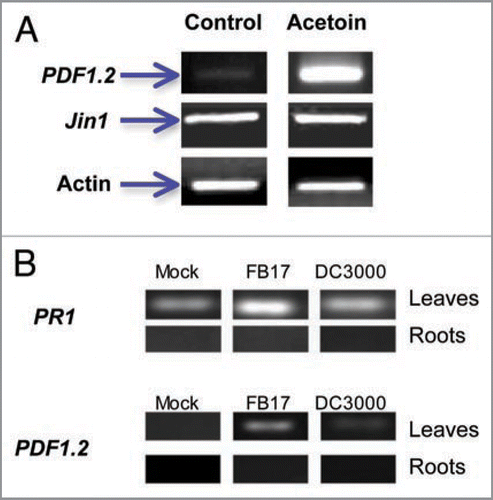
To further confirm that a FB17 component, acetoin, mediates this response, we analyzed the defense genes PDF1.2 and Jin1 whose expression depends on functional ET and JA pathways. Our RT-PCR data shows that acetoin and FB17 treated A. thaliana plants express more PDF1.2 and PR1 transcripts but not Jin1 in leaves compared to the mock inoculated plants (). Surprisingly, our results negated PDF1.2 and PR1 transcript expression in roots under FB17 treatments (). Further, co-inoculations of DC3000 with B. subtilis acetoin biosynthetic mutants (BSIP1173 and BSIP1174) also showed similar results confirming the role of NPR-1 (Suppl. Fig. 5). These results establish the involvement of this FB17 derived volatile component in mediating defense responses against DC3000 through NPR1 and ET dependent pathways by triggering PR1 and PDF1.2 expressions.
Discussion
Different rhizobacterial strains including B. subtilis have been previously reported to protect plants against a number of pathogens including viruses.Citation14,Citation20,Citation27,Citation34–Citation38 Our recent results showed that A. thaliana plants specifically recruit B. subtilis when experiencing an aerial pathogen attack from DC3000.Citation31 These results revealed for the first time that the inducible plant responses triggered by DC3000 pathogenesis include the induction of root secretions that effectively recruit B. subtilis in the rhizosphere.Citation31 In this study, root inoculation of A. thaliana with B. subtilis FB17 strain was found to stimulate plant defense pathways, though the elicitation profile of the genes involved was different from that previously reported using other rhizobacteria and biocontrol agents. It has been reported that B. subtilis root colonization protects plants against cucumber mosaic virus by a SA and NPR1 independent mechanism.Citation14,Citation27 Our analysis using FB17 and defense-compromised mutants of A. thaliana indicated that the resistance to DC3000 occurred via NPR1 and required SA/ET, whereas the Jar-1 was not essential. Although there are numerous examples of microorganisms interacting to trigger salubrious plant responses or a deleterious outcome, few reports have probed the role microbial VOCs may play in triggering biochemical changes of either primary or secondary plant metabolism. Results presented here indicate that a volatile bacterial component has the potential to induce systemic resistance in A. thaliana against DC3000 pathogenesis.
An important hallmark of the induction of systemic resistance is PR gene expression and the induction is signaled by the elevated levels of SA accumulated in the tissue showing systemic resistance.Citation39 Exogenous application of SA has been reported to induce systemic resistance and PR gene expression.Citation39–Citation41 Our results with the defense compromised mutants show that resistance to DC3000 mediated by FB17, occurred primarily through the NPR1 pathway and required SA and ET components. Both npr1-1 and NahG plants treated with FB17 negated any enhanced resistance against DC3000 pathogenesis. NahG mutants are unable to accumulate SA because the incorporated gene product salicylate hydroxylase converts SA to the inactive form catechol and, therefore, are unable to induce PR-1.Citation41 Similar results were obtained with the ET compromised mutant, etr1-3, which also showed no disease resistance against DC3000 post FB17 treatment. Interestingly, FB17 treatment of wild type A. thaliana plants induced accumulation of SA along with PR1 expression. Although our results indicate that resistance against DC3000 mediated by FB17 is NPR1 dependent and requires SA. Other PGPR strains such as P. fluorescens WCS417r reduced both the growth and symptom development by P. syringae in the leaves of A. thaliana upon root inoculation.Citation42 However, in this case the ISR was independent of SA accumulation and PR gene expression,Citation43 indicating that the mechanism to induce ISR is pathogen and PGPR strain specific. A recent study shows that a mixed inoculum treatment of B. subtilis and B. amyloliquefaciens triggers ISR through a SA dependent pathway against DC3000.Citation43 Further, understanding of the mechanisms of translation of ISR by B. subtilis into a real benefit for plants in terms of disease resistance could be an interesting aspect.
Our in vivo pathogenicity assay results showed a significantly reduced disease incidence, symptom development and DC3000 multiplication in the leaves of B. subtilis root colonized plants. The data mainly indicated that this protection is brought about by the reduced proliferation of the pathogen in the inoculated leaves of the B. subtilis root inoculated plants. This was supported by the significantly lower DC3000 CFUs per gram fresh weight of the inoculated leaves in the case of B. subtilis treated plants. However, like previous studiesCitation42,Citation43–Citation48 and our study, the introduction of B. subtilis and challenging plants with P. syringae are spatially separated. This ruled out the possibility of direct effect of acetoin on DC3000 by contact, and demonstrated that the effect was plant mediated. Additionally, exposing the plants uniformly with acetoin also ensured that the effect was systemic and not local. Although, previous studies showed a successful protection of plants from root fungal infectionsCitation49,Citation50 and aerial feeding by beetlesCitation46 no effort has been made to study the effect of B. subtilis induced ISR on the aerial bacterial infections in A. thaliana.
Previously, it was shown by Ryu et al.Citation14 that a volatile blend from B. subtilis GB03 and B. amyloliquefaciens IN937a was able to induce ISR in A. thaliana and reduce the disease severity of Pectobacterium carotovorum (formerly called as Erwinia carotovora subsp. Carotovora). The involvement of the B. subtilis volatile metabolite, acetoin, in the induction of systemic resistance was again confirmed in our study by treating the plants with acetoin. Interestingly, acetoin treatment triggered PR1 expressions and SA accumulation in A. thaliana plants. Acetoin treated A. thaliana plants revealed increased expressions for PDF1.2, suggesting that this compound targets SAR and ET pathways to upregulate defense responses in A. thaliana. These results also overlap with our defense compromised mutants study, wherein etr1-3, npr1-1 and NahG plants revealed no difference in disease symptoms and DC3000 CFUs between FB17 treated and untreated samples. Other metabolites such as surfactin and fengycin lipopeptides produced by B. subtilis strains induced systemic resistance in bean and tomato plants apart from activating the key enzymes of the lipoxygenase pathway in the resistant plants.Citation48 However the results with B. subtilis acetoin biosynthetic mutant strains which did not induce ISR compared to the control wild type strains, confirmed the involvement of acetoin in inducing ISR against DC3000. Higher bacterial multiplication and symptom development was observed in the case of the plants treated with acetoin biosynthetic mutants compared to plants treated with wild type strains FB17, GB03 and parental strain of the mutants BS168. However, the higher efficiency of FB17 observed in terms of ISR induction also indicates that there are might be additional mechanisms specific to FB17 compared to other strains such as GB03. These observations conclusively established the involvement of an exometabolite volatile (acetoin) in mediating disease protection in plants against P. syringae. However, the question related to involvement of a plant component that triggers acetoin biosynthesis in B. subtilis remains unanswered. There is a tempting possibility that root secretions may play a definite role in chemotaxis and elicitation of acetoin biosynthesis in B. subtilis. Our future line of studies in this direction will investigate the role of root exudates on induction of acetoin metabolism in B. subtilis. Some of the common plant metabolites such as organic acids, especially oxaloacetate, trigger the alsSD operon, required for acetoin production.Citation53
Although our results strongly suggest that the acetoin induced ISR restricts pathogen multiplication and disease development, the obvious question is whether this response was specific to B. subtilis? We addressed this question by employing another PGPR strain P. fluorescens (Pf01).Citation42 Interestingly, Pf01 root inoculated plants failed to show protection against DC3000 infection. In contrast, previous studies have reported that other P. fluorescens strains such as WCS417r induce ISR against P. syringae in A. thaliana.Citation42 These results suggest that few strains of P. fluorescens induce ISR against P. syringae also suggesting that the plant protection inflicted by this bacterial species is strain specific in nature.
In conclusion, our data show that protection of A. thaliana against DC3000 by a plant growth-promoting rhizobacterium follows a pathway that is dependent on NPR1 and requires SA and ET components but is independent of JA. Further confirmation was provided that FB17 derived VOC acetoin, induces the PDF1.2 and PR1 genes as a component of ET and SA signaling pathways, respectively. Evidence for SA-dependent but NPR1-independent pathways for regulation of PR1 gene expression and resistance to bacterial pathogens have been reported previouslyCitation51–Citation55 and we conclude that in our system a resistance, involving NPR1-SA dependent pathway is operational with the FB17 association. Although it was shown previously that the B. subtilis volatiles trigger an ISR response in A. thaliana through an ET dependent pathway against Erwinia pathogenesis,Citation14 the involvement of SA and ET pathways together to trigger ISR is not very commonly seen in plants. The ability to prime the plant for an augmented response to pathogen attack is expected to confer a fitness advantageCitation56 to the rhizobacterial-treated plants. This capacity to induce disease resistance in a versatile manner is expected to work in concert with their proven antibiotic effects and their colonization efficiency, making these rhizobacteria excellent candidates for effective biocontrol agents.
Materials and Methods
Plant material and chemicals.
A. thaliana wild type cultivar Columbia (Col-0) seeds were procured from Lehle Seeds (Round Rock, TX, and USA). The A. thaliana lines npr1-1, etr1-3, jar1-1 and NahG plants were obtained from Arabidopsis Biological Resource Center (ABRC), Ohio State University, Columbus, OH 43210 USA. Acetoin was obtained from Sigma-Aldrich, USA. Seeds were washed in double distilled water three times and surface sterilized using 50% commercial bleach (sodium hypochlorite) for 3–5 min followed by 3–4 washes in sterile distilled water. Seeds were cultured on Murashige and Skoog’sCitation57 (MS) solid medium with 3% sucrose and, allowed to germinate for 3–4 days at 23 ± 2°C 16 hr light/8 hr dark photoperiod until the roots and shoots emerged. The plates were illuminated with cool white fluorescent light with an intensity of 24 µmol m−2 s−1.
Bacterial cultures.
The strains of PGPR B. subtilis FB17 (obtained from Dr. Ray Fall, University of Colorado, Boulder), BS168, GBO3, BSIP1171, BSIP1173, BSIP1174 (from Dr. Paul Paré, Department of Chemistry and Biochemistry, Texas Tech University, Lubbock, TX 79409) were maintained on LB plates and P. fluorescens Pf01 and P. aeruginosa (PAO1) (obtained from Dr. George O’Toole, Department of Microbiology and Immunology Dartmouth Medical School, Hanover, NH 03755-3842). P. syringae DC3000 and E. coli (OP50) (obtained from Dr. Jorge M. Vivanco, Colorado State University, Fort Collins, CO 80523-1173) were maintained on LB plates with 50 µg ml−1 rifampicin. A single colony from a freshly streaked plate with or without antibiotic selection of each of the cultures was used to grow overnight cultures from which approximately OD600 = 0.02–0.05 culture was prepared and used in all the experiments. For routine plant based studies, cells were grown in LB medium at 37°C with shaking set at 220 rpm.
Effect of FB17 root inoculation on the P. syringae infection.
A. thaliana Col-0 plants grown on peat pellets in a growth chamber set for a photoperiod of 16 hr light and 8 hr dark at 23 ± 2°C and illuminated with cool white fluorescent light with an intensity of 24 µmol m−2 s−1 for a twenty day period. The plants were root inoculated with FB17 (OD600 = 0.5) by drenching with 10 ml of the culture in water. Fully expanded leaves were pressure inoculated with 100 µl of OD600 = 0.02 culture of P. syringae the next day. Different treatments included control (without DC3000 infiltration), control root inoculated with FB17, mock (leaf inoculated with sterile water), DC3000 (only leaf infiltration), DC3000 + FB17 (DC3000 leaf infiltration of FB17 root inoculated plants). The plants were transferred to magenta boxes and incubated in the growth chamber for an additional four days. The experiment was terminated after four days and the observations such as disease incidence (percentage of chlorotic leaves per plant), number of colony forming units per gram fresh weight of the leaf were recorded. Leaf samples were also collected and fixed for imaging the bacterial multiplication. Further, the co-cultivated roots were collected and fixed in 4% para-formaldehyde to image for B. subtilis binding and biofilm formation. Similar experiments using disease compromised A. thaliana mutants were carried out for FB17 treatment and DC3000 infections. Each treatment had at least 6 biological replicates and the experiment was repeated at two independent occasions.
Analysis of free salicylic acid (SA) content in the leaves of FB17 colonized plants.
Free SA content, in the leaves of plant roots inoculated with or without FB17 and also with or without leaf infiltration with DC3000, was estimated by following a modified protocol from Scott et al.Citation58 Briefly, leaf tissues (up to 0.1 g) were finely ground in liquid N2 and extracted overnight at 4°C in 5 ml 80% methanol. Samples were air dried at 25°C and re-dissolved in 10 ml of 0.2 M sodium acetate buffer (pH 4.5) and centrifuged (13,000 rpm, 5 min). After sample centrifugation, the supernatant was collected and the pH was adjusted to pH 2.0 with 0.1 M HCl and extracted with equal volume of ethyl acetate. The organic phase was back-washed against H2O and evaporated to dryness at 25°C. Samples were re-dissolved in 500 µl of methanol. The samples were analyzed by injecting 30 µl of the extract in to a Dynex Acclaim®, Polar Advantage II, C18 column (5 µm, 120 A°, 4.6 × 150 mm) and separated using a 0% to 100% gradient of methanol in 2 mM formic acid over 60 min. at a flow rate of 1 ml min−1 on a Dynex liquid chromatograph. The total free salicylic acid level was calculated by comparing the peak area from a known concentration of standard SA and the unknown sample. Final data was calculated and expressed as µM of SA per gram fresh weight. Free SA was also estimated with other bacterial root treatments such as Pf0-1, PAO1 and OP50 similar to the above-mentioned protocol. Free SA estimation of acetoin treated plants was performed post-exposure of plants to acetoin (1 ml of 10 mM stock). Each treatment had at least 6 biological replicates and the experiment was repeated at two independent occasions.
Effect of acetoin and acetoin mutants of B. subtilis on P. syringae infection and disease development on the leaves of Col-0.
All of the conditions for this experiment were similar to the above described, except that the additional treatments of root inoculation of Col-0 plants with wild type B. subtilis strains GBO3, FB17, BS168 and acetoin mutants BSIP1173 and BSIP 1174 were included. A separate set of treatments with acetoin as a volatile was generated by placing acetoin (1 ml of 10 mM stock) in a vial in the corner of the magenta box (Dimensions: 3 × 3 × 4″, growing area of 6.125 sq. inches Plantmedia Co.,) containing the plants with or without leaf infiltration by P. syringae was also set up and made airtight by sealing the cap with four coats of parafilm. All of the observations that were recorded for the earlier experiment were also recorded for this experiment. Each treatment had at least 6 biological replicates and the experiment was repeated at two independent occasions.
Effect of 2,3-butanediol on P. syringae infection and disease development on Col-0.
We used a previously established protocolCitation59 to analyze the effect of 2,3 butanediol in inflicting resistance against DC3000 in A. thaliana seedlings. The assays were performed in liquid media, wherein surface-sterilized A. thaliana (Col-0) seedlings (5 days old) were suspended in 24 well plates containing MS (1%) media supplemented with 2.5 mm 2-(N-morpholino) ethanesulfonic acid (MES), pH 5.8 (Sigma-Aldrich). Five-day-old seedlings were inoculated with P. syringae DC3000 at a final concentration of 1 × 107 CFU ml−1 (OD600 = 0.02). A total volume of 1 ml of 10 mM 2,3 butanediol was then added to the last column of a 96 well plate where the plants were placed in the first four columns for volatile treated plates (1 ml of ddH2O was added to control plates). Plates were continuously stirred at 80 rpm under continuous light at 22–25°C in a controlled environment room. Seedling phenotypes were assessed at 1–4 days post-inoculation using stereo microscope (Zeiss Axioskop-2), wherein, chlorophyll degradation with loss of red fluorescence was used as an indicator of DC3000 disease progression. To enumerate bacterial populations in inoculated seedlings in the liquid assay, seedlings were removed, rinsed for 20 seconds in 70% ethanol and were homogenized in 100 µl of ddH2O. After homogenization, tube volume was brought to 1 ml and bacteria were quantified by serial dilution plating.
Effect of acetoin on in vitro growth of P. syringae.
The effect of acetoin on P. syringae was studied by microdilution assay plates where 100 µl culture (OD600 = 0.02) was included into each well in alternative rows and every alternative row contained 100 µl acetoin (10 mM). The control plate received a similar quantity of culture and alternate rows received 100 µl of sterile water. The plates were incubated at 30°C on a rotary shaker. The OD600 was recorded in a plate reader at regular intervals up to 24 h. Each treatment had at least 6 biological replicates and the experiment was repeated at two independent occasions.
Effect of P. fluorescens root inoculation on the P. syringae infection and disease in the leaves.
All of the conditions for this experiment were the same as the experiments described above except that the plants were root inoculated with P. fluorescens Pf01 (OD600 = 0.5) by drenching with 10 ml of the culture in water. All the observations that were recorded for the earlier experiment were also recorded for this experiment. Each treatment had at least 6 biological replicates and the experiment was repeated at two independent occasions.
RT-PCR analysis of defense related genes.
Total RNA was extracted from leaves and roots of wild type plants after 12 h with or without FB17 treatment using RNeasy Plant Mini Kit (Qiagen, USA) according to the manufacturer’s instructions. cDNAs were synthesized from 300 ng of total RNA by using oligo dT primer (Ambion, Austin, TX) and Omniscript kit (Qiagen) according to manufacturer’s instruction. PCR amplification was carried out using oligonucleotide primers specific to A. thaliana Actin-2 (obtained from Sigma, USA Cat # C3615- 1SET) and PR1 (F = 5′ AGG TGC TCT TGT TCT TCC CTC GAA 3′; Reverse = 5′ TAC ACC TCA CTT TGG CAC ATC CGA 3′), PDF1.2 (F = 5′ GCT GCT CTT GTT CTC TTT GCT GCT 3′ Reverse = 5′ GGG ACG TAA CAG ATA CAC TTG TGT GC 3′) and Jin1 (F = 5′ ATG ACC CGA TTG GAA CAC CTG GAT 3′ Reverse = 5′ TGT CTC TCT GCT TCG ACG TGG TTT 3′) respectively. The PCR program used is as follows: denature at 95°C for 30 sec, anneal at 55°C for 20 sec, with an extension at 72°C for 1 min and a final extension at 72°C for 5 min.
Microscopy.
In order to view adherent FB17 cells and biofilm on the root surface by laser scanning confocal microscopy, the roots were stained with SYTO®13 (Invitrogen, Molecular Probes, Eugene, OR). Images were captured with a 10X Plan-Apochromat objective (numerical aperture 0.45) or 40X C-Apochromat objective (numerical aperture 1.2) on a Zeiss LSM 510 NLO attached to an Axiovert 200M with Zeiss AIM software (Rel. 3.2). Images were acquired with the 488 nm line excitation of an Argon laser using a 505 nm long pass emission filter. Experiments were performed 24 hours post-inoculation and post-treatment with FB17 (5 µl culture of OD600 = 0.02) to 10 day old plants grown in 4 ml liquid MS medium with 1% sucrose. All experiments were repeated twice with three replicates each.
Statistical analysis.
All of the data were averaged from two separate experiments unless mentioned otherwise and further analyzed for variance followed by a Student’s t test and ANOVA with the Benjamini-Hochberg correctionCitation60 for multiple testing when necessary using a statistical package JMP®7.0. The data means were considered significantly different at the probability of p ≤ 0.05.
Figures and Tables
Figure 1 Pseudomonas syringae DC3000 infection symptom development (A), bacterial multiplication (B), on the plants with or without root inoculation with B. subtilis FB17. The images are a representative sample of n = 6 and the data is an average of two separate experiments with six replicates. The yellowing of leaves in (A) shows classical DC3000 inflicted disease symptoms indicative of chlorosis. Different letters on the bars indicate a statistically significant difference (F(4,25) = 312.6, p ≤ 0.05, ANOVA).
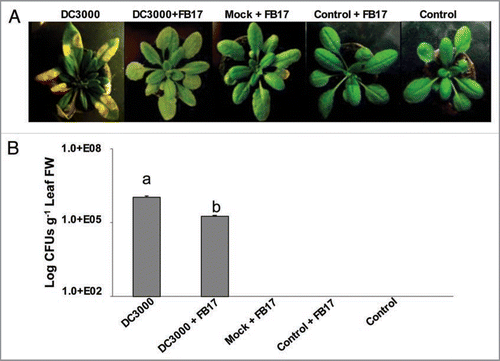
Figure 2 Effect of acetoin on disease symptom development (A), and pathogen proliferation (B) in the leaves of A. thaliana Col-0 plants leaf inoculated with DC3000. The images are a representative example of n = 6 and the data is an average of two separate experiments each with six replicates. The yellow patches on the leaves in (A), shows classical DC3000 inflicted disease symptoms in the form of chlorosis. Different letters on the bars indicate a statistically significant difference (p < 0.05, t-test).
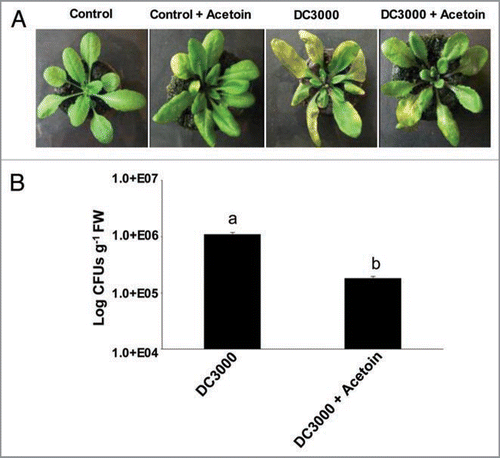
Figure 3 Effect of root colonization of B. subtilis acetoin biosynthetic mutants (A), on disease symptom development (B) percent disease incidence (C), and pathogen multiplication (D) in the leaves of A. thaliana Col-0 plants inoculated with DC3000. The images are a representative example of n = 6 and the data is an average of two separate experiments each with six replicates. The root confocal images were taken using 10x objective lens. The green fluorescence in (A) shows the FB17 biofilm on the root surface. The yellow patches on the leaves in the (B) show classical DC3000 inflicted disease symptoms in the form of chlorosis (scale bar: A = 100 µm). Different letters on the bars indicate a statistically significant difference between treatments (C: F(6,43) = 153.1, p < 0.05, ANOVA; D: F(6,43) = 125.3, p < 0.05, ANOVA).
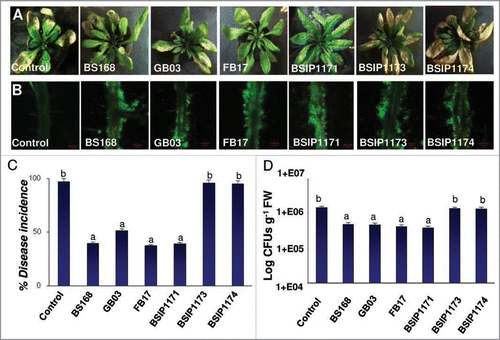
Figure 4 Defense-compromised A. thaliana plants revealing differential leaf colony titer response of DC3000 under B. subtilis FB17 (A) and acetoin treatment (B). Twenty one days old wild type and disease compromised mutants were root inoculated with FB17 and the leaves were infected with DC3000. The infected plant leaves were used post 96 hr of treatment to enumerate total leaf CFU counts for DC3000. The data is an average of six replicates of two experiments conducted (Mean ± S.D; n = 6). Different letters on the bars indicate a statistically significant difference (F(2,29) = 231.2, p ≤ 0.05, ANOVA).
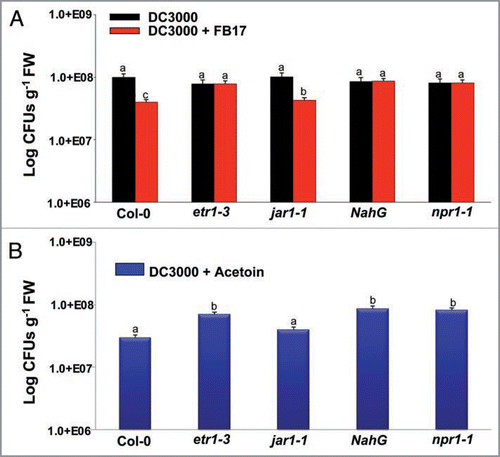
Figure 5 (A) Induction of ethylene and JA responsive genes (PDF1.2 & Jin1) 4 days after acetoin challenge. (B) Induction of PR1 and PDF1.2 genes in roots and leaves of FB17 treated plants. Plants were analyzed 4 days after FB17 challenge. Transcript levels were checked in both leaves and roots of mock, FB17 and DC3000 inoculated plants. Panels indicate the transcript levels for two genes (PR1 and PDF1.2) in both leaves and roots of plants. Column labels indicate that plants were mock treated (blunt infiltration in leaves and flooding roots with deionized water), followed by root inoculation with FB17 and infiltration of leaves with DC3000. The data is an average of six replicates of two experiments conducted separately and the images are a representative of six replicates.
Additional material
Download Zip (1.7 MB)Acknowledgements
H.P.B. acknowledges the support from University of Delaware Research Foundation (UDRF) and NSF Award IOS-0814477. P.W.P. acknowledges the partial financial support from Welch Foundation (Grant D-1478).
References
- Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 1998; 62:34 - 38
- Galán JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 1999; 284:1322 - 1328
- Hammerschmidt R. Phytoalexins: what have we learned after 60 years?. Annu Rev Phytopathol 1999; 37:285 - 306
- Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 2004; 198:249 - 266
- Cameron RK, Dixon RA, Lamb CJ. Biologically induced systemic acquired resistance in A. thaliana. Plant J 1994; 5:715 - 725
- Sticher L, Mauch-Mani B, Métraux JP. Systemic acquired resistance. Annu Rev Phytopathol 1997; 35:235 - 270
- Kniskern JM, Brian-Traw M, Bergelson J. Salicylic acid and jasmonic acid signaling defense pathways reduce natural bacterial diversity on Arabidopsis thaliana. Mol Plant Microbe Int 2007; 20:1512 - 1522
- Traw BM, Kniskern JM, Bergelson J. SAR increases fitness of Arabidopsis thaliana in the presence of natural bacterial pathogens. Evolution 2007; 61:2444 - 2449
- Ryals J, Uknes S, Ward E. Systemic acquired resistance. Plant Physiol 1994; 104:1109 - 1112
- Van Loon LC. Induced resistance in plants and the role of pathogenesis-related proteins. Eur J Plant Pathol 1997; 103:753 - 762
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, et al. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nature Genet 2000; 26:403 - 410
- Bais HP, Fall R, Vivanco JM. Biocontrol of Bacillus subtilis against infection of Arabidopsis thaliana root by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 2004; 134:307 - 319
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 2009; 63:541 - 556
- Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Paré PW. Bacterial volatiles trigger induced systemic resistance in Arabidopsis thaliana. Plant Physiol 2004; 134:1017 - 1026
- Van Peer R, Niemann GJ, Schippers B. Induced resistance and phytoalexin accumulation in biological control of fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology 1991; 81:728 - 734
- Wei G, Kloepper JW, Tuzun S. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology 1991; 81:1508 - 1512
- Maurhofer M, Hase C, Meuwly P, Métraux J-P, Défago G. Induction of systemic resistance of tobacco to tobacco necrosis virus by the root colonizing Pseudomonas fluorescens strain CHAO: Influence of the gacA gene and of pyoverdine production. Phytopathol 1994; 84:139 - 146
- Zhou T, Paulitz TC. Induced resistance in the biocontrol of Pythium aphanidermatum by Pseudomonas spp on cucumber. J Phytopathol 1994; 142:51 - 63
- Liu L, Kloepper JW, Tuzun S. Induction of systemic resistance in cucumber against bacterial angular leaf spot by plant growth-promoting rhizobacteria. Phytopathol 1995; 85:843 - 847
- Leeman M, den Ouden FM, van Pelt JA, Dirkx FPM, Steijl H, Bakker PAHM, Schippers B. Iron availability affects induction of systemic resistance to fusarium wilt of radish by Pseudomonas fluorescens. Phytopathol 1996; 86:149 - 155
- Kloepper JW, Ryu MN, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathol 2004; 94:1259 - 1266
- Dal-Soo K, Cook RJ, Weller DM. Bacillus sp L324-92 for biological control of three root diseases of wheat grown with reduced tillage. Biol Control 1997; 87:551 - 558
- Emmert EAB, Handelsman J. Biocontrol of plant disease: a Gram positive perspective. FEMS Microbiol Lett 1999; 171:1 - 9
- Bacon CW, Yates IE, Hinton DM, Meredith F. Biological control of Fusarium moneliforme in maize. Environ Health Persp 2001; 109:325 - 332
- Estevez de Jensen C, Percich JA, Graham PH. Integrated management strategies of bean root rot with Bacillus subtilis and Rhizobium in Minnesota Field. Crops Res 2002; 74:107 - 115
- Warrior P, Konduru K, Vasudevan P. Gnanamanickam SS. Formulation of biological control agents for pest and disease management. Biological control of crop diseases 2002; New York Marcel Dekker, 421 - 442
- Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW. Bacterial volatiles promote growth in Arabidopsis thaliana. Proc Natl Acad Sci, USA 2003; 100:4927 - 4932
- Farag MA, Ryu CM, Sumner LW, Pare PW. GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochem 2006; 67:2262 - 2268
- Arimura G, Ozawa R, Horiuchi J, Nishioka T, Takabayashi J. Plant-plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem Syst Ecol 2001; 29:1049 - 1061
- Gatehouse JA. Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 2002; 156:145 - 169
- Rudrappa T, Czymmek KJ, Paré PW, Bais HP. Root secreted malic acid recruits beneficial soil bacteria. Plant Physiol 2008; 148:1547 - 1556
- Ramos HC, Hoffmann T, Marco M, Hafed N, Presecan-Siedel E, Dreesen O, et al. Fermentative metabolism of Bacillus subtilis: Physiology and regulation of gene expression. J Bacteriol 2000; 182:3072 - 3080
- Rudrappa T, Quinn WJ, Stanley-Wall NR, Bais HP. A degradation product of the salicylic acid pathway triggers oxidative stress resulting in downregulation of Bacillus subtilis biofilm formation on Arabidopsis thaliana roots. Planta 2007; 226:283 - 297
- Raupach GS, Liu L, Murphy JF, Tuzun S, Kloepper JW. Induced systemic resistance in cucumber and tomato against cucumber mosaic cucumo-virus using plant growth promoting rhizobacteria (PGPR). Plant Dis 1996; 80:891 - 894
- Kloepper JW. Host specificity in microbe-microbe interactions. Biosci 1996; 46:14 - 18
- Zehnder GW, Yao C, Wei G, Kloepper JW. Influence of methyl bromide fumigation on microbe-induced resistance in cucumber. Biocontrol Sci Technol 2000; 10:687 - 693
- Murphy JF, Zehnder GW, Schuster DJ, Sikora EJ, Polston JE, Kloepper JW. Plant growth-promoting rhizobacterial mediated protection in tomato against Tomato Mottle Virus. Plant Dis 2000; 84:779 - 784
- Murphy JF, Reddy MS, Ryu C-M, Kloepper JW, Li R. Rhizobacteria-mediated growth promotion of tomato leads to protection against Cucumber mosaic virus. Phytopathol 2003; 93:1301 - 1307
- Uknes S, Winter AM, Delaney T, Vernooij E, Morse A, Friedrich L, et al. Biological induction of systemic acquired resistance in Arabidopsis thaliana. Mol Plant Microbe Interact 1993; 6:692 - 698
- Malamy J, Klessig DF. Salicylic acid and plant disease resistance. Plant J 1992; 2:643 - 654
- Lawton KA, Friedrich L, Hunt M, Weymann K, Delaney T, Kessmann H, et al. Benzothiadiazole induces disease resistance in Arabidopsis thaliana by activation of the systemic acquired resistance signal transduction pathway. Plant J 1996; 10:71 - 82
- Pieterse CMJ, van Wees SCM, Hoffland E, van Pelt JA, van Loon LC. Systemic resistance in Arabidopsis thaliana induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis related gene expression. Plant Cell 1996; 8:1225 - 1237
- Ryu CM, Murphy JF, Reddy MS, Kloepper JW. A two-strain mixture of rhizobacteria elicits induction of systemic resistance against Pseudomonas syringae and cucumber mosaic virus coupled to promotion of plant growth on Arabidopsis thaliana. J Microbiol Biotechnol 2007; 17:280 - 286
- van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 1998; 36:453 - 483
- Wei G, Kloepper JW, Tuzun S. Induced systemic resistance to cucumber diseases and increased plant growth by plant growth-promoting rhizobacteria under field conditions. Phytopathol 1996; 86:221 - 224
- Zehnder GW, Kloepper JW, Tuzun S, Yao C, Wei G, Chambliss O, Shelby R. Insect feeding on cucumber mediated by rhizobacteria-induced plant resistance. Entomologia Experimentalis et Applicata 1997; 83:81 - 85
- Kloepper JW, Tuzun S, Zehnder GW, Wei G. Multiple disease protection by rhizobacteria that induce systemic resistance-historical precedence. Phytopathol 1997; 87:136 - 137
- Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, et al. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol 2007; 9:1084 - 1090
- Schilling O, Frick O, Herzberg C, Ehrenreich A, Heinzle E, Wittmann C, Stülke J. Transcriptional and metabolic responses of Bacillus subtilis to the availability of organic acids: transcription regulation is important but not sufficient to account for metabolic adaptation. Appl Environ Microbiol 2007; 73:499 - 507
- Radjacommare R, Ramanathan A, Kandan A, Harish S, Thambidurai G, Sible GV, et al. PGPR mediates induction of pathogenesis-related (PR) proteins against the infection of blast pathogen in resistant and susceptible ragi [Eleusine coracana (L) Gaertner] cultivars. Plant and Soil 2004; 266:165 - 176
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis thaliana expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 1997; 9:1573 - 1584
- Clarke JD, Liu Y, Klessig DF, Dong X. Uncoupling PR gene expression from NPR1 and bacterial resistance: characterization of the dominant Arabidopsis thaliana cpr6-1 mutant. Plant Cell 1998; 10:557 - 569
- Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT. The gain-of-function Arabidopsis thaliana acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses and cell growth. Plant Cell 1999; 11:1695 - 1708
- Devadas SK, Enyedi A, Raina R. The Arabidopsis thaliana hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signaling in cell death and defence against pathogens. Plant J 2002; 30:467 - 480
- Rairdan GJ, Delaney TP. Role of salicylic acid and NIM1/NPR1 in race-specific resistance in Arabidopsis thaliana. Genetics 2002; 161:803 - 811
- Van Hulten M, Pelser M, Van Loon LC, Pieterse CMJ, Ton J. Costs and benefits of priming for defense in Arabidopsis thaliana. Proc Natl Acad Sci USA 2006; 103:5602 - 5607
- Murashige T, Skoog F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Planta 1962; 15:473 - 497
- Scott IM, Clarke SM, Wood JE, Mur LAJ. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis thaliana. Plant Physiol 2004; 135:1040 - 1049
- Schreiber K, Ckurshumova W, Peek J, Desveaux D. A high-throughput chemical screen for resistance to Pseudomonas syringae in Arabidopsis. Plant J 2008; 54:522 - 531
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 1995; 57:289 - 300