Abstract
GABAA receptors mediate the majority of fast synaptic inhibition in the mammalian brain. Mechanisms that regulate GABAA function are thus of critical importance in modulating overall synaptic inhibition. Phosphorylation of GABAA receptor subunits is one such mechanism that leads to the dynamic modulation of GABAA receptor function. In particular, phosphorylation of tyrosine residues 365 and 367 (Y365, Y367) within the γ2 subunit of GABAA receptors has been shown in previous in vitro studies to negatively regulate clathrin-dependent endocytosis of GABAA receptors and to enhance the efficacy of synaptic inhibition. With the aim of investigating the impact of this phosphorylation-dependent modulation of GABAA receptors on animal behavior, we recently developed a knock-in mouse in which these critical tyrosine residues within the γ2 subunit have been mutated to phenylalanines (Y365/7F). These animals exhibited enhanced GABAA receptor accumulation at postsynaptic inhibitory synaptic specializations on pyramidal neurons within the hippocampus, primarily due to aberrant trafficking within the endocytic pathway. We found that this enhanced inhibition correlated with a specific deficit in spatial memory in these mice, without modifying a number of other behavioral paradigms. Here, we summarize our recently reported observations and further discuss their possible implications.
GABAA receptors are heteropentameric chloride-selective ligand-gated ion channels that mediate fast synaptic inhibition in the adult central nervous system. Regulation of the number of GABAA receptors at synapses is critical for maintaining the correct level of synaptic inhibitory transmission and physiological function.Citation1 Recent studies have suggested that dysregulation in GABAA receptor trafficking may underlie pathophysiological conditions, including pharmaco-resistance and self-sustaining seizures in status epilepticus,Citation2,Citation3 increased excitotoxicity in ischemiaCitation4 and trait anxiety.Citation5 Understanding the endogenous mechanisms that regulate GABAA receptor membrane trafficking is thus of clinical significance.
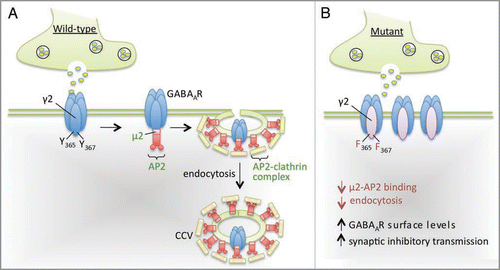
GABAA receptor trafficking can be regulated, in part, by the controlled removal of receptors from the membrane in clathrin-coated vesicles. Endocytosis of GABAA receptors is facilitated by the clathrin adaptor protein 2 (AP2) complex, which interacts directly with neuronal GABAA receptor subunits (). A classical tyrosine-based (Yxxφ) binding motif for AP2 is located within the intracellular domain of the γ2 subunit of the GABAA receptor, centered on tyrosine residues 365 and 367.Citation6,Citation7 This motif binds the γ2 subunit of the AP2 complex with highaffinity.Citation6 Consistent with studies of other tyrosine-based internalization motifs,Citation8 the tyrosine residues Y365/7 in the γ2 subunit can be phosphorylated by Src family tyrosine kinases.Citation9–Citation11 Phosphorylation of these residues has been demonstrated in prior in vitro studies to negatively regulate clathrin-dependent endocytosis of GABAA receptors and to enhance the efficacy of synaptic inhibition.Citation6,Citation9,Citation10
In our recent study,Citation12 we aimed to investigate the impact of this phosphorylation- dependent modulation of GABAA receptors on animal behavior. We used a genetic-engineering approach to generate a knock-in mouse in which tyrosine residues 365 and 367 within the GABAA receptor γ2 subunit were mutated to phenylalanines. Surprisingly, homozygous mice for this mutation exhibited a lethal phenotype, with morbidity occurring at a very early embryonic developmental stage. One intriguing speculation is that GABAA receptor signaling plays a key role in neurogenesis/ early developmental maturation of the nervous system, however, further studies will be needed to determine the exact underlying cause of the embryonic lethality.
We then focused our study on male mice heterozygous for the γ2(Y365/7F) mutation. We observed a dramatic reduction in the level of tyrosine phosphorylation of the γ2 subunit in γ2(Y365/7F), which was an expected result based on the mutation of these critical tyrosine phosphorylation sites. We also observed significant increases in the cell surface expression levels of the γ2 subunit in the hippocampus of heterozygous mice, an effect that was mediated by a reduction in the level of endocytosis of the mutant γ2 subunit. Thus, Y365/7 residues play a central role in regulating GABAA receptor endocytosis in vivo. This is consistent with our in vitro studies demonstrating that mutation of these tyrosine residues to phenylalanines significantly reduces binding to μ2-AP2Citation6,Citation12 ().
One interesting observation was that the increase in γ2 subunit levels in the mutants was not uniform throughout the hippocampus. It appeared to be particularly dominant in specific neuronal populations, such as CA3 pyramidal neurons. The reasons underlying this region-specific disparity are currently unknown, however it will be an interesting question to address in future work. Differences in the subunit composition of GABAA receptors between the different hippocampal subregions may be one underlying cause.Citation13 Regional specificity in the particular tyrosine kinases and/or phosphatases that regulate phosphorylation of endogenous γ2 subunits may also contribute to the differential regulation of GABAA receptor trafficking within distinct hippocampal subregions.
We next assessed whether these region-specific increases in the γ2 subunit expression had an impact on hippocampal-dependent behavior. After confirming that the γ2(Y365/7F) heterozygotes have no deficits in basal locomotor or exploratory behavior, we then assessed the cognitive abilities of these mice. We did this by using the spatial version of the object recognition task, which is based on the ability of animals to discriminate between familiar and novel spatial locations of objects. Strikingly, the mutant mice failed to discriminate between the displaced and nondisplaced objects, whereas wild-type mice displayed selectivity for the newly displaced object. This difference was not due to a deficit in object recognition memory, since the mutants performed similarly to wild-type mice when subjected to the novel object recognition task. Thus, our behavioral studies demonstrate that γ2(Y365/7F) heterozygote animals have specific deficits in spatial memory, a task that is critically dependent on the CA3 region of the hippocampus.Citation14 Moreover, this behavioral deficit is mirrored by an enhancement in the efficacy of synaptic inhibition in CA3, which presumably underlies this behavioral deficit.Citation12 This result is reminiscent of animal models of Down syndrome, where excessive levels of neuronal inhibition are believed to contribute to cognitive deficits.Citation15
The possibility of improving memory performance and cognition by altering the balance between excitatory and inhibitory neurotransmission is a working hypothesis that is currently being investigated by many groups for its therapeutic potential.Citation16 For example, a reversal of the cognitive impairments observed in a mouse model of Down syndrome was achieved by pharmacological treatment with GABAA receptor antagonists.Citation15 Other studies have proposed that partial inverse agonists with a preference for α5-containing GABAA receptors may be particularly good candidates for memory-enhancing drugs.Citation17,Citation18 Thus, drugs that target specific GABAA receptor populations, or alternatively, pharmacological manipulation of endogenous GABAA receptor trafficking mechanisms, may prove useful for achieving cognitive enhancement in the near future.
In summary, our results demonstrate that two tyrosine residues (Y365/7) within the GABAA receptor γ2 subunit play a critical role in shaping hippocampal neuronal activity by regulating the cell surface accumulation of GABAA receptors at inhibitory synapses and consequently the efficacy of synaptic inhibition. Mutation of these residues results in aberrant synaptic GABAA receptor trafficking and dysfunctional hippocampal-dependent learning. Based on our results, and recent results from other investigators, it thus appears that alterations in the number and/or function of GABAA receptors can have significant effects on memory and cognition.
Figures and Tables
Figure 1 Model summarizing the regulation of GABAA receptor endocytosis by the Yxxφ motif within the GABAA receptor γ2 subunit. (A) Endocytosis via clathrin-coated vesicles (CCV) is the major internalization mechanism for neuronal GABAA receptors (GABAARs). The intracellular domain of the γ2 subunit of the GABAAR interacts with the μ2 subunit of the clathrin-adaptor protein 2 (AP2). This binding is mediated via a Yxxφ motif contained within the γ2 subunit that is centered around tyrosine residues 365 and 367. (B) Binding of the AP2 complex is negatively regulated by mutation of these tyrosine residues to phenylalanines. γ2(Y365/7F) heterozygous mutant mice have decreased levels of receptor endocytosis and increased cell-surface levels of γ2-containing GABAARs. We have also demonstrated that these mutant mice display an enhanced efficacy of inhibitory synaptic transmission in the CA3 region of the hippocampus.Citation12
Acknowledgements
S.J.M. is supported by National Institute of Neurological Disorders and Stroke Grants (NS047478, NS051195, NS056359, NS054900), the Medical Research Council, and the Wellcome Trust. R.J. is supported by the Maltz Family Foundation as a National Alliance for Research on Schizophrenia and Depression Young Investigator.
Addendum to:
References
- Jacob TC, Moss SJ, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 2008; 9:331
- Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005; 25:7724
- Terunuma M, et al. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci 2008; 28:376
- Mielke JG, Wang YT. Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABA receptors following oxygen-glucose deprivation in cultured cortical neurons. J Neurochem 2005; 92:103
- Crestani F, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci 1999; 2:833
- Kittler JT, et al. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor γ2 subunit. Proc Natl Acad Sci USA 2008; 105:3616
- Smith KR, et al. Regulation of inhibitory synaptic transmission by a conserved atypical interaction of GABAA receptor β- and γ-subunits with the clathrin AP2 adaptor. Neuropharmacology 2008; 55:844
- Prybylowski K, et al. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron 2005; 47:845
- Moss SJ, Gorrie GH, Amato A, Smart TG. Modulation of GABAA receptors by tyrosine phosphorylation. Nature 1995; 377:344
- Valenzuela CF, et al. Tyrosine kinase phosphorylation of GABAA receptors. Brain Res Mol Brain Res 1995; 31:165
- Brandon NJ, Delmas P, Hill J, Smart TG, Moss SJ. Constitutive tyrosine phosphorylation of the GABAA receptor γ2 subunit in rat brain. Neuropharmacology 2001; 41:745
- Tretter V, et al. Deficits in spatial memory correlate with modified γ-aminobutyric acidA receptor tyrosine phosphorylation in the hippocampus. Proc Natl Acad Sci USA 2009; 106:20039
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 1995; 359:154
- Stupien G, Florian C, Roullet P. Involvement of the hippocampal CA3-region in acquisition and in memory consolidation of spatial but not in object information in mice. Neurobiol Learn Mem 2003; 80:32
- Fernandez F, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci 2007; 10:411
- Fernandez F, Garner CC. Over-inhibition: a model for developmental intellectual disability. Trends Neurosci 2007; 30:497
- Chambers MS, et al. Identification of a novel, selective GABAA α5 receptor inverse agonist which enhances cognition. J Med Chem 2003; 46:2227
- Mohler H, et al. Regulation of cognition and symptoms of psychosis: focus on GABAA receptors and glycine transporter 1. Pharmacol Biochem Behav 2008; 90:58