Abstract
Ras proteins are laterally segregated into transient nanoclusters on the plasma membrane, a property essential for high fidelity signal transduction through the MAPK pathway. From a proteomic screen we identified nucleophosmin (NPM) and nucleolin as two novel regulators of K-Ras plasma membrane interactions that in turn influence MAP Kinase signaling. NPM and nucleolin are predominately nucleolar proteins but also possess extra-nuclear functions. We showed that a subset of NPM and nucleolin localize to the inner leaflet of the plasma membrane and specifically interact with K-Ras but not H-Ras. This interaction is independent of the activation state of K-Ras, and stabilizes K-Ras membrane levels. NPM expression also increases the fraction of K-Ras in nanoclusters. The increase in clustered K-Ras-GTP enhances signaling through the MAPK pathway. Together these results identify NPM and nucleolin as a new class of K-Ras regulators that modulate signal transduction via the MAPK pathway.
Ras proteins control a diverse range of cellular processes including cell proliferation, differentiation and survival. Ras proteins are small GTPases that function as molecular switches on the plasma membrane transmitting signals from cell surface receptors to the cell interior. They are key modulators of the MAP Kinase (MAPK) pathway, delivering high fidelity signal transmission.Citation1–Citation3 Critical residues in the C-terminal hypervariable region (HVR) confer correct localization and isoform specific function on Ras proteins. The minimal membrane anchors of K-Ras, H-Ras and N-Ras together with flanking sequences and the G-domain, determine Ras interactions with lipids and proteins on the inner leaflet of the plasma membrane, leading to isoform-specific signaling output.Citation4,Citation5 Dysregulation of Ras signaling results in abnormal cell growth and tumor formation. Mutations are most prevalent in the K-Ras gene, and are associated with pancreatic, colon and non-small cell lung cancer.Citation6,Citation7
To further understand K-Ras mediated functions, we undertook a proteomic screen to identify proteins that specifically interact with K-Ras. This study revealed two nucleolar proteins, nucleophosmin (NPM) and nucleolin, as specific K-Ras interacting proteins.Citation8 Co-immunoprecipitation analysis confirmed that NPM and nucleolin specifically bind to K-Ras but not H-Ras in the cell. Domain deletion experiments showed that the C-terminal polybasic region of K-Ras binds to the N-terminal domain of NPM,Citation8 excluding a trivial explanation that the interaction between K-Ras and NPM is due to electrostatic interactions in vitro between the NPM acidic domain and the K-Ras basic domain. NPM and nucleolin are predominately localized to the nucleolus but have also been reported to have extra-nuclear functions.Citation9–Citation11 Importantly, using electron microscopy, we definitively showed a pool of NPM and nucleolin localizing to the inner leaflet of the plasma membrane. Compared to the nucleolar pool of NPM and nucleolin, the subset of NPM and nucleolin on the plasma membrane was in considerably lower abundance. Nonetheless, we show that K-Ras, NPM and nucleolin formed a complex in the membrane fraction of cells.Citation8
This poses the interesting question of what is the role of the NPM, nucleolin, K-Ras complex on the plasma membrane. Our data demonstrate that recruitment of NPM and nucleolin to the plasma membrane are K-Ras independent events. In contrast, overexpression of NPM and nucleolin both led to increased overall cellular K-Ras protein levels. This also correlated with an increase in plasma membrane bound K-Ras.Citation8 Furthermore, we observed increased levels of both GTP and GDP loaded K-Ras on the membrane. The interaction between K-Ras and NPM is significantly enhanced if farnesylation of K-Ras is blocked by point mutation.Citation8 In intact cells therefore NPM may preferentially interact with membrane-bound, rather than cytosolic K-Ras since the farnesyl moiety that inhibits the interaction will be partitioned into the lipid bilayer.
On the plasma membrane Ras proteins occupy distinct dynamic domains called nanoclusters that are critical for functional activity and essential for high fidelity signaling through the MAPK pathway.Citation1–Citation3 The spatial distribution of K-Ras is modulated by its activation state and recent work shows that K-Ras-GTP nanoclusters are spatially distinct from K-Ras-GDP nanoclusters. Citation12–Citation14 Strikingly, our data showed that in addition to stabilizing K-Ras levels on the membrane, overexpression of NPM also increased the clustered fraction of both GTP and GDP bound K-Ras, but not the size of the nanoclusters. Nucleolin expressed alone did not alter the extent of K-Ras clustering, nor did co-expression potentiate the enhanced clustering induced by NPM. Therefore, the roles of nucleolin and NPM in modulating K-Ras function appear to be distinct. One possibility is that nucleolin operates as a chaperone that facilitates delivery of K-Ras to the PM, whereas NPM operates exclusively at the plasma membrane to drive K-Ras clustering. In this context the increase in K-Ras plasma membrane levels stimulated by nucleolin would reflect increased transport. In contrast the increase in K-Ras levels stimulated by NPM would be a direct result of increased nanoclustering, since nanoclustered K-Ras is more stably associated with the plasma membrane than monomeric K-Ras.Citation15
Other scenarios however cannot be excluded. For example, nucleolin has recently been shown to interact with the cytoplasmic domain and modulate the function of ErbB receptors.Citation16,Citation17 It is possible that these activities direct ErbB signaling through K-Ras. Whatever the molecular mechanisms, consistent with the effects on K-Ras plasma membrane levels and nanoclustering, expression of NPM or nucleolin alone or in combination increases ERK activation.Citation8 In sum, our data shows that a subset of NPM and nucleolin bind and stabilize K-Ras on the inner leaflet of the plasma membrane. The increase in K-Ras levels on the membrane is synchronized by NPM with an increase in the clustered fraction of K-Ras, both GTP and GDP loaded. Elevated K-Ras-GTP nanoclusters lead to increased activation of ERK ().
To date, the major scaffold protein shown to associate with K-Ras on the plasma membrane is Galectin-3 (Gal-3).Citation15,Citation18 The interaction between Gal-3 and K-Ras is prenyl dependent and is restricted to K-Ras-GTP nanoclusters; moreover Gal-3 is recruited from the cytosol to the membrane by K-Ras-GTP.Citation18 In contrast, NPM is constitutively associated with the membrane, and interacts with both GDP and GTP bound K-Ras.Citation8 The significant differences between Gal-3 and NPM suggest that NPM is a novel class of regulator of K-Ras plasma membrane interactions. The interplay between Gal-3 and NPM regulation of K-Ras is likely critical for control of the MAPK pathway. Many details remain to be worked out. We favor the idea that plasma membrane level of NPM sets a basal level of K-Ras clustering, whereas the cytosolic levels of Gal-3 determine the clustered fraction of K-Ras-GTP after K-Ras activation. We have speculated that modulating the basal level of K-Ras clustering may have a role in driving MAPK activation during M phase of cell cycle, when extranuclear levels of NPM increase with breakdown of the nuclear envelope.Citation8 In contrast, elevated levels of cytosolic Gal-3, as occur in many malignancies will increase the number of K-Ras-GTP nanoclusters for any given amount of K-Ras-GTP, amplifying MAPK output.Citation15
It is also important to highlight that like Gal-3, NPM and nucleolin are overexpressed in many cancers.Citation19 In particular, 35% of adult acute myeloid leukemia cases have a frameshift mutation in the NPM gene that results in aberrant redistribution of NPM to the cytoplasm.Citation20,Citation21 Based on our results, one intriguing possibility is that the overexpressed and/or mislocalized NPM partitions to the plasma membrane leading to increased K-Ras nanoclustering and elevated K-Ras signaling. Therefore it is imperative we understand the mechanism of NPM and nucleolin targeting and how it correlates with oncogenesis.
Figures and Tables
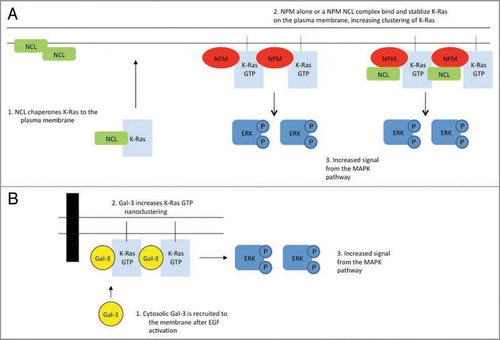
Figure 1 Model for the role of NPM, nucleolin and Gal-3 on K-Ras function. (A) Nucleolin (NCL) may operate as a chaperone to facilitate delivery of K-Ras to the plasma membrane. NPM specifically interacts with K-Ras on the plasma membrane. NCL also interacts with K-Ras on the plasma membrane probably in a complex with NPM. NPM and NCL stabilize K-Ras plasma membrane levels. This may be a consequence of increased delivery and increased nanoclustering in the case of NCL and NPM respectively. NPM alone, or NPM in complex with NCL increases the amount of K-Ras-GDP and K-Ras-GTP in nanoclusters. Increased clustering of K-Ras-GTP leads to increased ER K activation. Note that NCL and NPM are not recruited to the plasma membrane by K-ras-GTP or K-rasGDP. (B) In contrast, cytosolic Gal-3 is recruited to the plasma membrane after EGF stimulation increases K-ras-GTP levels. Recruited Gal-3 drives K-Ras-GTP nanoclustering, which leads to increased ER K activation. Whether Gal-3 nanoclusters also contain NPM and or NCL remains to be determined.
Acknowledgements
This work was supported by award R01GM066717 from the National Institute of General Medical Sciences. J.F.H. is the current incumbent of the Fondren Chair in Cellular Signaling.
Addendum to:
References
- Harding A, Tian T, Westbury E, Frische E, Hancock JF. Subcellular localization determines MAP kinase signal output. Curr Biol 2005; 15:869 - 873
- Inder K, Harding A, Plowman SJ, Philips MR, Parton RG, Hancock JF. Activation of the MAPK module from different spatial locations generates distinct system outputs. Mol Biol Cell 2008; 19:4776 - 4784
- Tian T, Harding A, Inder K, Plowman S, Parton RG, Hancock JF. Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat Cell Biol 2007; 9:905 - 914
- Abankwa D, Hanzal-Bayer M, Ariotti N, Plowman SJ, Gorfe AA, Parton RG, et al. A novel switch region regulates H-ras membrane orientation and signal output. EMBO J 2008; 27:727 - 735
- Hancock JF, Parton RG. Ras plasma membrane signalling platforms. Biochem J 2005; 389:1 - 11
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res 1989; 49:4682 - 4689
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 2003; 3:11 - 22
- Inder KL, Lau C, Loo D, Chaudhary N, Goodall A, Martin S, et al. Nucleophosmin and nucleolin regulate K-Ras plasma membrane interactions and MAPK signal transduction. J Biol Chem 2009; 284:28410 - 28419
- Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 1989; 56:379 - 390
- Ginisty H, Amalric F, Bouvet P. Nucleolin functions in the first step of ribosomal RNA processing. EMBO J 1998; 17:1476 - 1486
- Szebeni A, Olson MO. Nucleolar protein B23 has molecular chaperone activities. Protein Sci 1999; 8:905 - 912
- Plowman SJ, Muncke C, Parton RG, Hancock JF. H-ras, K-ras and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc Natl Acad Sci USA 2005; 102:15500 - 15505
- Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol 2001; 3:368 - 375
- Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol 2003; 160:165 - 170
- Plowman SJ, Ariotti N, Goodall A, Parton RG, Hancock JF. Electrostatic interactions positively regulate K-Ras nanocluster formation and function. Mol Cell Biol 2008; 28:4377 - 4385
- Di Segni A, Farin K, Pinkas-Kramarski R. Identification of nucleolin as new ErbB receptors-interacting protein. PLoS ONE 2008; 3:2310
- Farin K, Di Segni A, Mor A, Pinkas-Kramarski R. Structure-function analysis of nucleolin and ErbB receptors interactions. PLoS One 2009; 4:6128
- Shalom-Feuerstein R, Plowman SJ, Rotblat B, Ariotti N, Tian T, Hancock JF, Kloog Y. K-ras nanoclustering is subverted by overexpression of the scaffold protein galectin-3. Cancer Res 2008; 68:6608 - 6616
- Lim MJ, Wang XW. Nucleophosmin and human cancer. Cancer Detect Prev 2006; 30:481 - 490
- Falini B, Bolli N, Shan J, Martelli MP, Liso A, Pucciarini A, et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood 2006; 107:4514 - 4523
- Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352:254 - 266