Abstract
It has long been claimed that human emotional expressions, such as laughter, have evolved from nonhuman displays. The aim of the current study was to test this prediction by conducting acoustic and phylogenetic analyses based on the acoustics of tickle-induced vocalizations of orangutans, gorillas, chimpanzees, bonobos, and humans. Results revealed both important similarities and differences among the various species’ vocalizations, with the phylogenetic tree reconstructed based on these acoustic data matching the well-established genetic relationships of great apes and humans. These outcomes provide evidence of a common phylogenetic origin of tickle-induced vocalizations in these taxa, which can therefore be termed “laughter” across all five species. Results are consistent with the claims of phylogenetic continuity of emotional expressions. Together with observations made on the use of laughter in great apes and humans, findings of this study further indicate that there were two main periods of selection-driven evolutionary change in laughter within the Hominidae, to a smaller degree, among the great apes and, most distinctively, after the separation of hominins from the last common ancestor with chimpanzees and bonobos.
Researchers have long noted that emotional expressions in humans and displays in nonhuman primates can be similar in both form and context,Citation1 leading many to suggest common phylogenetic origins. Citation2–Citation5 Human smiling and laughter have received much of the attention,Citation6–Citation8 with the case for homologies strengthened by evidence of strong cross-species similarities in the production anatomy of both facialCitation9,Citation10 and vocalCitation11 expressions. The current work focused on laughter in particular, testing the hypothesis of phylogenetic continuity as directly as possible by measuring the acoustics of tickling-induced vocalizations in humans and all four great ape species, and then submitting the results to quantitative phylogenetic analyses.Citation12
Laughter was deemed a strong candidate for phylogenetic reconstruction, as these sounds are deeply grounded in human biology.Citation13,Citation14 Vocalizations referred to as “laughter” also occur in great apes engaged in tickling and social play.Citation5,Citation7,Citation15 Vettin and TodtCitation16 have shown key similarities in the respective acoustics of play- and tickling-induced vocalizations in juvenile chimpanzees (Pan troglodytes) and tickling-induced laughter in adult humans.
The current work investigated tickling-induced laughter and vocalizations in humans and apes, based on collecting and analyzing these sounds from all four great ape species for the first time. The goal was to situate the origin and evolution of laughter within the larger phylogeny of the Hominidae by using obtained acoustic data as raw material, first for cross-species acoustic comparisons and then for quantitative phylogenetic reconstructions. The phylogenetic analyses of this study notably differed from earlier approaches. While vocal data previously helped to reconstruct the phylogeny of species and populations,Citation17–Citation19 the present study used the already well-established phylogeny of humans and great apes as a reference to measure the evolutionary relationship of vocal expressions. In all, 21 infant and juvenile orangutans (Pongo pygmaeus), gorillas (Gorilla gorilla), chimpanzees and bonobos (P. paniscus) were recorded as they were tickled by familiar humans. In addition, laugh recordings were obtained from three human infants who were tickled by their mothers. For representative spectrograms, see .
Statistical comparisons based on the acoustic data indicated important similarities and differences among the species in 11 spectral, temporal and airflow variables (for variable definitions, see and Davila Ross et al.Citation20 Table S2). The most distinctive acoustic differences were found between humans and the great apes as a group. Humans produced sounds that were more voiced (with regular vocal-fold vibration), with more vibration regimes (patterns of energy distribution over time), and more egressive airflow (vocalized while exhaling) than did the apes. Among the great apes, orangutans produced the longest calls and longest inter-call intervals. Orangutans and gorillas also showed fewer calls per vocalization series and more egressive calls than chimpanzees and bonobos. Phylogenetic analyses based on the acoustic data replicated the already well-established genetic relationships among the five species, placing humans closest to bonobos and chimpanzees, more distant from gorillas, and furthest from orangutans (two exhaustive searches with the maximum-parsimony method, treelength = 110–113, retention index = 0.686–0.750). The resulting trees indicated well-resolved topologies and strong support for the associated clades (bootstrap value of 79–97%). Acoustic characters found to be most important to phylogenetic trees with the most consistent directional changes (highest retention index values) were the number of vibration regimes per call, call duration and the number of calls per series.
Taken together, the acoustic and phylogenetic results of this study provide evidence of common ancestry for laughter in humans and tickling-induced vocalizations in great apes and, consequently, support the more general claim of phylogenetic continuity from nonhuman displays to human emotional expressions.Citation1–Citation5 “Laughter” therefore is not an anthropomorphic term, and can instead arguably be traced as a vocalization type back to at least the last common ancestor of modern great apes and humans, approximately ten to sixteen million years ago.Citation21,Citation22
The data of the present study further suggest that the distinctive characteristics of human laughter, such as voicing and airflow direction, emerged from preexisting acoustic traits. While speech-related selection could have been a driving force for these changes,Citation23 it should also be noted that there are key differences in the occurrence of laughter between humans and great apes. Whereas apes laugh primarily in the contexts of social play and tickling, laughter of humans occurs across a wide range of contexts.Citation24,Citation25 This dramatic expansion of laughter production most likely occurred in an intermediate hominin species, suggesting that selection could have been acting directly on these sounds. Notably, human infants also produce whoops, pleasure cries and hics when tickled,Citation26 vocalizations that seem to be absent in the apes.
A close relationship between acoustic-and context-related differences in laughter also seems to be present between the Asian and African great apes. In addition to producing the most distinctive laughter among the great apes, orangutans clearly were the species to laugh least during the tickling sessions, instead they predominantly produced squeaks.Citation27 This behavioural pattern seems to extend to social play as well.Citation8,Citation16,Citation27–Citation29 In other words, all tickle-induced vocalizations occurring among non-human primates cannot a priori be considered homologous to human laugh sounds. By extension, the evolutionary relation between primate laughter and tickle-induced calls produced by other species such as flying foxes (Pteropus conspicillatus, see video supplement) and ratsCitation30 should also be specifically tested using acoustically based phylogenetic analyses before conclusions are drawn concerning potential homologies.
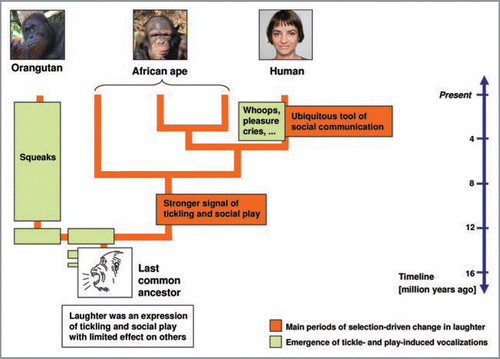
This seemingly strong link between the form and function of laughter across great apes and humans suggests that the phylogenetic changes of laugh acoustics implicated in the current work were primarily a product of adaptation. We suggest that there were likely two main kinds of change in laughter acoustics and behaviour over the past ten to sixteen million years (see ). Based on evidence of production differences in orangutans versus the other species, we first infer that laughter occurring in the common ancestor of great apes and humans was limited in usage and effect. Whereas laughter in ancestral African apes became a more prevalent and effective acoustic signal and the predominant vocalization of play, squeaks assumed this role in orangutans. We further suggest that after the separation of the hominins from their common ancestor with chimpanzees and bonobos, laughter underwent even greater change, expanding beyond its origins in tickling and play contexts to become a ubiquitous, acoustically distinctive signalling tool occurring in almost every conceivable form of human social communication.
Figures and Tables
Figure 1 Representative spectrograms (40-ms Hanning window) of tickle-induced vocalizations from four great ape species and humans. Recordings had a 22,050-Hz sampling rate. This illustration first appeared as in Davila Ross et al.Citation20
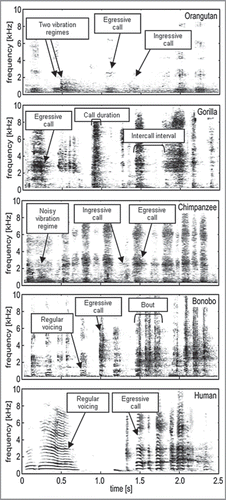
Figure 2 Model of the evolution of laughter and other vocalizations of tickling and play in great apes and humans. Two main periods of acoustic and function-related changes in laughter were likely to have occurred within the past ten to sixteen million years while other tickle- and play-induced vocalizations evolved. It remains unknown whether laughter and squeaks emerged prior to or in the common ancestor of great apes and humans. Notably, lesser apes produce tickle-induced vocalizations that acoustically resemble orangutan laughter (e.g., Symphalangus syndactylus: Davila Ross et al.Citation20; Hylobates lar: Zimmermann pers. obs.) and squeak-like calls during play (Nomascus spp.: Thomas Geissmann, pers. comm.). The figure is adapted from Davila Ross et al.Citation20 Figure 4.
Additional material
Download Zip (2.1 MB)Acknowledgements
Thanks go to Hugh Spencer (Cape Tribulation Tropical Research Station) for providing the video material. The study was funded by the University of Veterinary Medicine Hannover and the Center for Systems Neuroscience Hannover. Acoustic analysis was partly supported by the Center for Behavioral Neuroscience under the STC Program of the National Science Foundation under Agreement No. IBN-9876754 and by the Brains & Behavior Initiative and Neuroscience Institute at Georgia State University. Travel costs were funded by Forschungszentrum Jülich and Freundeskreis der Tierärztlichen Hochschule Hannover.
Addendum to:
References
- Darwin C. The expression of emotion in man and animals 1872; London, UK Murray
- Andrew RJ. The origin and evolution of the calls and facial expressions of the primates. Behaviour 1963; 20:1 - 107
- Redican WK. Rosenblum L. Facial expressions in nonhuman primates. Primate behavior: Developments in field and laboratory research 4 1975; New York, NY Academic Press 103 - 194
- van Hooff JARAM. Facial expressions in higher primates. Symp Zool Soc Lond 1962; 8:67 - 125
- van Lawick-Goodall J. The behaviour of free-living chimpanzees in the Gombe Stream area. Anim Behav Monogr 1968; 1:65 - 311
- Chevalier-Skolnikoff S. Ekman P. Facial expression of emotion of nonhuman primates. Darwin and facial expression: A century of research in review 1973; New York, NY Academic Press 11 - 89
- Preuschoft S, van Hooff JARAM. Homologizing primate facial displays: A critical review of methods. Folia Primatol 1995; 65:121 - 137
- van Hooff JARAM. Hinde RA. A comparative approach to the phylogeny of laughter and smiling. Non-verbal communication 1972; Cambridge, UK Cambridge University Press 209 - 241
- Sherwood CC, Holloway RL, Erwin JM, Schleicher A, Zilles K, Hof PR. Cortical orofacial motor representation in old world monkeys, great apes and humans: I. Quantitative analysis of cytoarchitecture. Brain Behav Evol 2004; 63:61 - 81
- Vick S-J, Waller BM, Parr LA, Smith Pasqualini MC, Bard KA. A cross-species comparison of facial morphology and movement in humans and chimpanzees using the facial action coding system (FACS). J Nonverbal Behav 2007; 31:1 - 20
- Fitch WT. The evolution of speech: A comparative review. Trends Cogn Neurosci 2000; 4:258 - 267
- Cavalli-Sforza LL, Edwards AWF. Phylogenetic analysis: Models and estimation procedures. Evolution 1967; 21:550 - 570
- Eibl-Eibesfeldt I. Human ethology 1989; New York, NY Aldine Transaction
- Makagon MM, Funayama SE, Owren MJ. An acoustic analysis of laughter produced by congenitally deaf and normally hearing college students. J Acoust Soc Am 2008; 124:472 - 483
- Provine RR. Laughter: A scientific investigation 2000; New York, NY Viking Press
- Vettin J, Todt D. Human laughter, social play and play vocalizations of non-human primates: An evolutionary approach. Behaviour 2005; 142:217 - 240
- Davila Ross M, Geissmann T. Call diversity of wild male orangutans: A phylogenetic approach. Am J Primatol 2007; 69:305 - 324
- Mendez-Cardenas M, Randrianambinina B, Rabesandratana A, Rasoloharijaona S, Zimmermann E. Geographic variation in loud calls of sportive lemurs (Lepilemur ssp.) and their implications for conservation. Am J Primatol 2008; 70:1 - 11
- Zimmermann E. Differentiation of vocalizations in bushbabies (Galaginae, Prosimiae, Primates) and the significance for assessing phylogenetic relationships. Z Zool Syst Evolutionsforsch 1990; 28:217 - 239
- Davila Ross M, Owren MJ, Zimmermann E. Reconstructing the evolution of laughter in great apes and humans. Curr Biol 2009; 19:1106 - 1111
- Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H, Shoshani J, et al. Towards a phylogenetic classification of primates based on DNA evidence complemented by fossil evidence. Mol Phylogenet Evol 1998; 9:585 - 598
- Stauffer RL, Walker A, Ryder OA, Lyons-Weiler M, Blair Hedges S. Human and ape molecular clocks and constraints on paleontological hypotheses. J Hered 2001; 92:469 - 474
- Gould SJ, Vrba ES. Exaptation—a missing term in the science of form. Paleobiology 1982; 8:4 - 15
- Gervais M, Wilson DS. The evolution and functions of laughter and humor: A synthetic approach. Q Rev Biol 2005; 80:395 - 430
- Owren MJ, Bachorowski J-A. Mayne TJ, Bonanno GA. The evolution of emotional expression: A “selfish-gene” account of smiling and laughter in early hominids and humans. Emotions: Current issues and future directions 2001; New York, NY The Guilford Press 152 - 191
- Scheiner E, Hammerschmidt K, Jürgens U, Zwirner P. Acoustic analyses of developmental changes and emotional expression in the preverbal vocalizations of infants. J Voice 2002; 16:509 - 529
- Davila Ross M. Towards the evolution of laughter 2009; Saarbruecken, DE Südwestdeutscher Verlag für Hochschulschriften Aktiengesellschaft
- de Waal FBM. The communicative repertoire of captive bonobos (Pan paniscus) compared to that of chimpanzees. Behaviour 1988; 106:183 - 251
- Schenkel R. Zur Ontogenese des Verhaltens bei Gorilla und Mensch. Z Morph Anthrop 1964; 54:233 - 259
- Panksepp J. Neuroevolutionary sources of laughter and social joy: Modeling primal human laughter in laboratory rats. Behav Brain Res 2007; 182:231 - 244