Abstract
The presence of polyploid neurons in the vertebrate nervous system has been a subject of debate since the 1960s. At that time, Purkinje cells were proposed to be tetraploid, but technical limitations impeded to reach a clear conclusion, and the current believe is that most vertebrate neurons are diploid. By using up-to-date approaches we have recently demonstrated the existence of a subpopulation of tetraploid retinal ganglion cells (RGCs) in the vertebrate retina. In the chick, these neurons show large somas and extensive dendritic trees and most of them express a marker specific of RGCs innervating a specific lamina of the optic tectum. We have also demonstrated that these neurons are generated in response to nerve growth factor (NGF) acting through the neurotrophin receptor p75 (p75NTR), which induces E2F1 activity and cell cycle re-entry in migrating RGC neuroblasts lacking retinoblastoma (Rb) protein. We have also showed that brain-derived neurotrophic factor (BDNF) prevents G2/M transition in the tetraploid RGCs, thus being crucial for the maintenance of the tetraploid status as well as the survival of these neurons. The realization that tetraploid neurons can be readily observed in the vertebrate nervous system has important physiological consequences, which are discussed in this commentary.
Several eukaryotes are known to undergo endoreduplicative cycles leading to somatic polyploidy, thus increasing cell size in specific tissues.Citation1,Citation2 Examples of polyploidy in neurons are known in some invertebrates.Citation3,Citation4 In contrast, the vertebrate nervous system has classically been thought to be constituted by neurons with a diploid DNA amount.Citation5 This belief was challenged when Lowell W. Lapham postulated in 1968 that Purkinje cells were tetraploid.Citation6 Following this initial observation, several authors claimed that other large vertebrate neurons were also tetraploid.Citation7–Citation9 Nevertheless, many other studies questioned this concept,Citation10 and the absence of reliable procedures for DNA quantification made it impossible to reach a clear conclusion at that time.Citation10
Modern techniques such as flow cytometry, fluorescent in situ hybridization (FISH), slide-based cytometry (SBC) and quantitative PCR analysis of DNA from isolated nuclei can all reliably quantify the amount of nuclear DNA in neurons.Citation11 By using SBC, flow cytometry and FISH, we have recently demonstrated that tetraploid neurons exist in the normal vertebrate retina, representing a subpopulation of RGCs that, in the chick, innervate lamina F in the stratum-griseum-et-fibrosum-superficiale of the tectal cortex.Citation12 These neurons are generated during the early stages of retinal development, soon after they acquire neuronal markers. Indeed, a subset of migrating RGCs expressing the transcription factor E2F1, and lacking Rb protein, was observed to undergo S-phase and remain in a permanent G2-like state. Therefore, endoreduplication, but not alternative mechanisms such as aneuploidy or cell fusion,Citation13,Citation14 represents the mechanism generating tetraploid RGCs in the vertebrate nervous system.
Our work has demonstrated that endoreduplication in RGCs is triggered by NGF through p75NTR, since blocking antibodies against these molecules were able to prevent cell cycle re-entry and tetraploidy in the retinal neurons.Citation12 Furthermore, activation of p75NTR in differentiating retinal neurons was observed to induce E2F1 activity.Citation12 These results are consistent with the observed decrease of BrdU incorporation described in the developing retina of p75NTR knock-out mice.Citation15 As far as we know this is the first time in which a signaling mechanism inducing endoreduplication in vertebrates has been revealed.
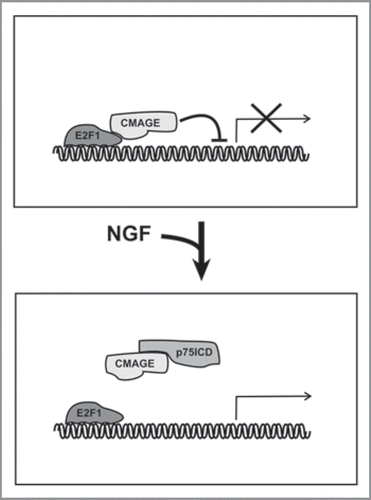
Both NGF and p75NTR are known to fulfill several functions in the nervous system including cell cycle regulation,Citation16 and several intracellular interactors of p75NTR have been shown to modulate cell cycle progression.Citation16 Among them, different members of the Melanoma Antigen (MAGE) protein family have been shown to mimic the E2F1-blocking effect of Rb,Citation17 thus being putative regulators of the postmitotic state of RGCs lacking Rb expression. CMAGE, the chicken MAGE protein, is known to co-localize with p75NTR in the developing RGCs,Citation17 thus representing a candidate protein for the regulation of endoreduplication in these neurons. p75NTR can release its intracellular domain (p75ICD) in response to ligand binding by means of a γ-secretase-dependent mechanism,Citation18 and the p75ICD peptide has been shown to facilitate E2F1 activity by preventing the blocking effect of CMAGE.Citation17 This suggests that endoreduplication may result from the activation of a p75ICD-containing peptide that subsequently blocks CMAGE, thus facilitating E2F1 function in postmitotic RGCs lacking Rb expression (). This model is further supported by a recent report demonstrating that p75ICD can interact with the promoter of cyclin E1,Citation19 a known cell cycle regulator crucial for endoreduplication,Citation1,Citation2 which is expressed in response to E2F1 activation.
Endoreduplication is characterized by DNA synthesis in the absence of cell division. In this regard, we have shown that most migrating RGCs that reactivate the cell cycle remain in a G2-like state.Citation12 The neurotrophin BDNF is likely involved in preventing G2/M transition in tetraploid RGCs since it has been shown to inhibit cyclin B1 expression and ectopic mitotic figures in NGF-treated, differentiating retinal cells.Citation20 Blockade of BDNF in embryonic eye explants resulted in an increase of differentiating RGCs undergoing mitosis,Citation12 thus indicating that endogenous levels of BDNF prevent G2/M transition in tetraploid RGCs. The mechanism used by BDNF to achieve this effect is currently unknown.
Differentiating tetraploid RGCs that undergo mitosis finally die.Citation12 This conclusion was evidenced by means of BrdU pulse and chase experiments in vivo, which allowed us to analyze the fate of migrating neuroblasts that undergo cell cycle re-entry. While most of these cells remained in the retina with a 4C DNA content, a minority of them were observed to undergo mitosis followed by apoptosis, as previously observed in vitro.Citation20 This finding indicates that the induction of apoptosis by p75NTR in the RGC neuroblastsCitation21 depends on the completion of mitosis in these cells, as previously suggested.Citation20 At present, the molecular mechanism triggering apoptosis in RGCs that undergo mitosis remains to be determined.
Tetraploid RGCs in the chick show increased soma size and extensive dendritic trees,Citation12 thus sharing features with primate parasol cells and α-Y cells in the cat.Citation22 These neurons are involved in motion processing and show a specific distribution pattern along the ganglion cell layer (GCL). Therefore, adjusting the numbers of large vs. small RGCs may be crucial to assure the proper functioning of the retina. In this regard, the density of large RGCs is known to be increased in the peripheral retina,Citation23 whereas apoptosis becomes increased in the center of this tissue.Citation24 This suggests that early cell death25 in the central retina is required to reduce the number of tetraploid RGCs and increase the ratio between small and large RGCs in this region. TGFβ might participate in the removal of tetraploid RGCs as it has been shown to cooperate with NGF in triggering retinal apoptosis,Citation26 mainly acting on large RGCs.Citation27
Cell cycle re-entry in neurons has classically been a synonym of apoptosis.Citation28 Our data indicate that reactivation of the cell cycle can also be interpreted in terms of neuronal tetraploidy. This extends the significance of cell cycle re-entry in neurodegeneration since it may also result in tetraploidization,Citation11 followed by neuronal hypertrophy,Citation29 which may result in altered neuronal function and changes in neuronal circuits leading to neuronal degeneration.Citation30
Figures and Tables
Figure 1 A hypothetical model for the mechanism used by NGF/p75NTR to trigger cell cycle re-entry, based on available data. Upper. E2F1 is inhibited by CMAGE in RGCs lacking Rb protein, thus maintaining their postmitotic state. Lower. The activation of p75NTR by NGF results in the presence of the intracellular domain of p75NTR (p75ICD) in the nucleus, which subsequently interacts with CMAGE and prevents its blocking effect on E2F1.Citation17
Addendum to:
References
- Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: more for less. Cell 2001; 105:297 - 306
- Ullah Z, Lee CY, Lilly MA, DePamphilis ML. Developmentally programmed endoreduplication in animals. Cell Cycle 2009; 8:1501 - 1509
- Coggeshall RE, Yaksta BA, Swartz FJ. A cytophotometric analysis of the DNA in the nucleus of the giant cell, R-2, in Aplysia. Chromosoma 1970; 32:205 - 212
- Manfredi Romanini MG, Fraschini A, Bernocchi G. DNA content and nuclear area in the neurons of the cerebral ganglion in Helix pomatia. Ann Histochim 1973; 18:49 - 58
- Swift H. Quantitative aspects of nuclear nucleoproteins. Int Rev Cytol 1953; 2:1 - 76
- Lapham LW. Tetraploid DNA content of Purkinje neurons of human cerebellar cortex. Science 1968; 159:310 - 312
- Herman CJ, Lapham LW. DNA content of neurons in the cat hippocampus. Science 1968; 160:537
- Herman CJ, Lapham LW. Neuronal polyploidy and nuclear volumes in the cat central nervous system. Brain Res 1969; 15:35 - 48
- Museridze DP, Svanidze IK, Macharashvili DN. Content of DNA and dry weight of the nuclei of neurons of the external geniculate body and retina of the eye in guinea pigs. Sov J Dev Biol 1975; 5:269 - 272
- Swartz FJ, Bhatnagar KP. Are CNS neurons polyploid? A critical analysis based upon cytophotometric study of the DNA content of cerebellar and olfactory bulbar neurons of the bat. Brain Res 1981; 208:267 - 281
- Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci 2007; 27:6859 - 6867
- Morillo SM, Escoll P, de la Hera A, Frade JM. Somatic tetraploidy in specific chick retinal ganglion cells induced by nerve growth factor. Proc Natl Acad Sci USA 2010; 107:109 - 114
- Kingsbury MA, Yung YC, Peterson SE, Westra JW, Chun J. Aneuploidy in the normal and diseased brain. Cell Mol Life Sci 2006; 63:2626 - 2641
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature 2002; 416:545 - 548
- Harada C, Harada T, Nakamura K, Sakai Y, Tanaka K, Parada LF. Effect of p75NTR on the regulation of naturally occurring cell death and retinal ganglion cell number in the mouse eye. Dev Biol 2006; 290:57 - 65
- López-Sánchez N, Frade JM. Control of the cell cycle by neurotrophins: lessons from the p75 neurotrophin receptor. Histol Histopathol 2002; 17:1227 - 1237
- López-Sánchez N, González-Fernández Z, Niinobe M, Yoshikawa K, Frade JM. Single mage gene in the chicken genome encodes CMage, a protein with functional similarities to mammalian type II Mage proteins. Physiol Genom 2007; 30:156 - 171
- Frade JM. Nuclear translocation of the p75 neurotrophin receptor cytoplasmic domain in response to neurotrophin binding. J Neurosci 2005; 25:1407 - 1411
- Parkhurst CN, Zampieri N, Chao MV. Nuclear localization of the p75 neurotrophin receptor intracellular domain. J Biol Chem 2009; PMID:20022966
- Frade JM. Unscheduled re-entry into the cell cycle induced by NGF precedes cell death in nascent retinal neurones. J Cell Sci 2000; 113:1139 - 1148
- Frade JM, Rodríguez-Tébar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature 1996; 383:166 - 168
- Crook JD, Peterson BB, Packer OS, Robinson FR, Troy JB, Dacey DM. Y-cell receptive field and collicular projection of parasol ganglion cells in macaque monkey retina. J Neurosci 2008; 28:11277 - 11291
- Naito J, Chen Y. Morphologic analysis and classification of ganglion cells of the chick retina by intracellular injection of Lucifer Yellow and retrograde labeling with DiI. J Comp Neurol 2004; 469:360 - 376
- Frade JM, Bovolenta P, Martínez-Morales JR, Arribas A, Barbas JA, Rodríguez-Tébar A. Control of early cell death by BDNF in the chick retina. Development 1997; 124:3313 - 3320
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci 2000; 23:454 - 458
- Dünker N, Schuster N, Krieglstein K. TGFbeta modulates programmed cell death in the retina of the developing chick embryo. Development 2001; 128:1933 - 1942
- Beier M, Franke A, Paunel-Görgülü AN, Scheerer N, Dünker N. Transforming growth factor beta mediates apoptosis in the GCL during all programmed cell death periods of the developing murine retina. Neurosci Res 20 2006; 56:193 - 203
- Becker EB, Bonni A. Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol 2004; 72:1 - 25
- Iacono D, Markesbery WR, Gross M, Pletnikova O, Rudow G, Zandi P, et al. The Nun study: clinically silent AD, neuronal hypertrophy and linguistic skills in early life. Neurology 2009; 73:665 - 673
- Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci 2001; 21:2661 - 2668