Abstract
Lipids may affect synaptic function in at least two ways: by acting as ligands for effector proteins (e.g. phosphatidylinositol (4,5) bisphosphate, diacylglycerol-mediated signalling) or by modifying the physicochemical properties and molecular organisation of synaptic membranes. One that acts in the latter manner is cholesterol, an essential structural component of plasma membranes that is largely enriched in the membranes of synapses and synaptic vesicles, in which it may be involved in lipid-lipid and protein-lipid interactions. Cholesterol is an important constituent of the “membrane rafts” that may play a role in recruiting and organising the specific proteins of the exocytic pathways. Furthermore, many synaptic proteins bind directly to cholesterol. The regulation of cholesterol and lipid levels may therefore influence the specific interactions and activity of synaptic proteins, and have a strong impact on synaptic functions.
Neurotransmitter release at neuronal synapses is mediated by synaptic vesicle exo-endocytosis, a process that involves the complex interplay of a specialized set of proteins including soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, scaffolding and cytoskeletal elements, and ion channels.Citation1 Work done over the last 12 years has shown that membrane lipid composition and distribution may play an important role in synaptic activity via the regulation of protein activity or distribution. Lipid-lipid and lipid-protein interactions may be required to mediate the recruitment of trafficking/tethering proteins at specific fusion/scission sites during vesicle cycling. Furthermore, certain lipids may control membrane shape, fluidity and curvature, which also have a major impact on synaptic vesicle exo-endocytosis.
Cholesterol and Cholesterol-Binding Proteins in Synapses
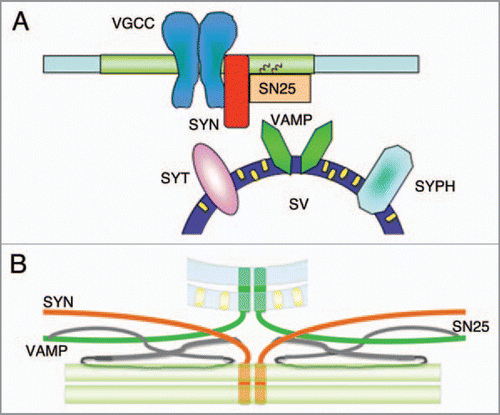
Cholesterol is an essential element of neural cell membranes. Cholesterolcholesterol/sphingolipid-rich microdomains have been found in the membranes of synapses, where they influence the stability and organization of a number of supramolecular protein complexes. These microdomains also contain proteins of the exo- and endocytic machinery, including t-SNARE proteins, syntaxin 1a and SNAP-25, and other proteins of the exocytic machinery, such as presynaptic P/Q calcium channelsCitation2–Citation5 (). These domains are cholesterol dependent, but there is conflicting evidence as to whether they contain significant amounts of sphingolipids and can be considered classical lipid rafts.
In addition to revealing the exact lipid composition of the plasma membrane microdomains containing proteins of the exocytic complex, recent studies have shown that cholesterol is enriched in the membrane of synaptic vesicles, where it accounts for about 30% of total lipids (a concentration of 215 ng/mg of protein) and is the most abundant lipid after phospholipids.Citation6 Furthermore, it is known that synaptic vesicle proteins such as synaptophysin and synaptotagmin bind cholesterolCitation7 (and Rosa P, unpublished observations).
On the basis of these findings, it is expected that modulating cholesterol levels would affect neurotransmitter release by influencing various aspects of synapse activity. Recent studies have shown that reducing cholesterol levels by means of treatment with a squalene synthase inhibitor (or zaragozic acid) decreases the synaptic bouton uptake of FM1-43 and anti-synaptotagmin1 antibodies. Most importantly, in synaptic boutons, the depolarization-induced increase in the fluorescence intensity of synapto-pHluorin (a recombinant form of the green fluorescent protein used to monitor exocytosisCitation8) is significantly reduced in cholesteroldepleted neurons, although the number of synaptic vesicles in the synapses is not altered. In addition, the reduction in antisynaptotagmin1 antibody uptake induced by zaragozic acid is almost completely counteracted by cholesterol reloading. Taken together, these findings demonstrate the involvement of cholesterol in exocytosisCitation9 and are consistent with previous findings of a large reduction in evoked synaptic transmission in methyl-β-cyclodextrin-treated crayfish neuromuscular junctions and hippocampal neurons.Citation10,Citation11 In line with the above, it can be hypothesized that cholesterol depletion may affect the assembly of exocytic complexes on presynaptic membranes. Furthermore, although the structural rigidity of cholesterol reduces membrane fluidity and hinders their deformation, there is evidence that it may accelerate membrane fusion. This may partially depend on a spontaneous negative curvature of cholesterol that makes it prefer to be in the negatively curved (concave) membrane rather than on the positively curved (convex) surface. One function of cholesterol in synaptic vesicle exocytosis may therefore be related to the direct modulation of membrane curvature.
Recent studies have found a link between cholesterol, membrane curvature and SNARE protein structure in a cell-free assay using large unilamellar vesicles containing t- or v-SNAREs and varying concentrations of cholesterol,Citation12 and it has been speculated that, by maintaining a favorable membrane curvature, physiological concentrations of cholesterol may help the transmembrane domains of v-SNARE protein synaptobrevin/VAMP-2 dimers to reach a conformation that is suitable for fusion with t-SNAREs (). In line with this role of maintaining the correct conformation of transmembrane VAMP-2 dimers, a significant amount of this v-SNARE has been recovered in detergent resistant membranes (thought to represent lipid rafts in live cells) sensitive to treatment with squalene synthase inhibitor.Citation9 In conclusion, various data from cell-free and intact cell assays strongly suggest that cholesterol influences synaptic vesicle exocytosis and synapse activity.
Figures and Tables
Figure 1 (A and B) Synaptic vesicle exocytosis and lipid microdomains. Schematic diagrams of synaptic protein distribution in cholesterol-enriched domains: it is thought that t-SNARE proteins (syntaxin 1, SYN; SNAP-25, SN25) and P/Q voltage-gated calcium channels (VGCCs) are organized in plasma membrane lipid microdomains, in which synaptic vesicle fusion may occur. On the other hand, synaptic vesicle membranes are rich in cholesterol (small yellow rectangles), which bind to synaptic vesicle proteins (synaptophysin, SYPH; synaptotagmin SYT) and may regulate membrane curvature and, very importantly, the alignment of the dimers of synaptobrevin1,2/VAMP-1,2 (VAMP) transmembrane domains in a parallel configuration that favours the formation of SNARE complexes and exocytosis.
Acknowledgements
This study was supported by grants from the Italian Consiglio Nazionale delle Ricerche, the Ministero Istruzione Università e Ricerca (MIUR) and Fondazione Cariplo.
Addendum to:
References
- Südhof TC. Neurotransmitter release. Handb Exp Pharmacol 2008; 184:1 - 21
- Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci USA 2001; 98:5619 - 5624
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, et al. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J 2001; 20:2202 - 2213
- Taverna E, Saba E, Rowe J, Francolini M, Clementi F, Rosa P. Role of lipid microdomains in P/Qtype calcium channel (CaV2.1) clustering and function in presynaptic membranes. J Biol Chem 2004; 279:5127 - 5134
- Lang T. SNARE proteins and membrane rafts. J Physiol 2007; 585:693 - 698
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riede D, et al. Molecular anatomy of a trafficking organelle. Cell 2 2006; 17:831 - 846
- Thiele C, Hannah MJ, Fahrenholz F, Huttner WB. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat Cell Biol 2000; 2:42 - 49
- Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 1 1998; 394:192 - 195
- Linetti A, Fratangeli A, Taverna E, Valnegri P, Francolini M, Cappello V, et al. Cholesterol reduction impairs exocytosis of synaptic vesicles. J Cell Sci 2010; 123:595 - 605
- Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol 2007; 579:413 - 429
- Zamir O, Charlton MP. Cholesterol and synaptic transmitter release at crayfish neuromuscular junctions. J Physiol 2006; 571:83 - 99
- Tong J, Borbat PP, Freed JH, Shin YK. A scissors mechanism for stimulation of SNARE-mediated lipid mixing by cholesterol. Proc Natl Acad Sci USA 2009; 106:5141 - 5146