Abstract
Alcohol abuse is a pervasive problem known to be influenced by genetic factors, yet our understanding of the mechanisms underlying alcohol addiction is far from complete. Drosophila melanogaster has been established as a model for studying the molecular mechanisms that mediate the acute and chronic effects of alcohol. However, the Drosophila model has not yet been extended to include more complex alcohol-related behaviors such as self-administration. We recently established a paradigm to characterize ethanol consumption and preference in flies. We demonstrated that flies prefer to consume ethanol-containing food over regular food, and this preference exhibits several features of alcohol addiction: flies increase ethanol consumption over time, they consume ethanol to pharmacologically relevant concentrations, they will overcome an aversive stimulus in order to consume ethanol, and they exhibit relapse after a period of ethanol deprivation. Thus, ethanol preference in flies provides a new model for studying important aspects of addiction and their underlying mechanisms. One mutant that displayed decreased ethanol preference, krasavietz, may represent a first step toward uncovering those mechanisms.
Alcohol abuse is a widespread medical and societal problem, with an estimated prevalence of 8.5% in the US.Citation1 Genetic factors contribute to one’s risk for alcoholism, but few specific genes have been identified.Citation2 Although alcoholism is an exclusively human phenomenon (for example, the DSM-IV criteria for alcohol abuse refer to legal, social and interpersonal problems related to alcoholCitation3), rodent models that represent specific aspects of alcohol addiction have provided valuable insight. The two-bottle choice paradigm, perhaps the most common ethanol assay used in rodents, measures an animal’s voluntary intake of ethanol solution relative to water.Citation4 Other assays such as conditioned place preference and operant conditioning measure the rewarding and reinforcing properties of ethanol, respectively.Citation4 Selective breeding,Citation5 pharmacological manipulations,Citation6 and reverse genetic approachesCitation7 using rodents have made significant contributions to our understanding of the mechanisms underlying ethanol preference. However, rodents are not the ideal model organism for unbiased, forward genetic approaches to identify novel genes involved in ethanol preference due to the significant time and expense required for genetic screening.
In contrast, Drosophila melanogaster is one of the most genetically accessible model organisms. Behavioral responses to ethanol are conserved between flies and mammals: both exhibit locomotor stimulation at low doses and motor incoordination and sedation at high doses.Citation8 Flies also exhibit tolerance with repeated ethanol exposures.Citation9,Citation10 Importantly, several molecular pathways shown to mediate acute responses to ethanol in flies, such as the cAMP,Citation11 neuropeptide F (neuropeptide Y in mammals),Citation12 and EGFRCitation13 pathways, also regulate mammalian ethanol responses.Citation13–Citation15
In addition to displaying mammalian-like responses to acute ethanol exposure, flies exhibit preference for ethanol in a number of measures: (1) females prefer to lay eggs on ethanol-containing substrates,Citation16 (2) larvae spend more time on ethanolcontaining agarCitation17 and (3) flies preferentially consume ethanol-containing food.Citation18 These preference behaviors likely reflect the evolutionary history of the fruit fly. In its natural environment, Drosophila melanogaster frequently encounters significant levels of ethanol produced by fermenting plant materials.Citation8 Flies have evolved mechanisms to process the ethanol they ingest, by efficiently degrading it for use as an energy source or a precursor for lipid biosynthesis.Citation8
Despite the significant progress in using flies to study the molecular mechanisms that mediate ethanol intoxication, little is known about the mechanisms underlying ethanol preference behaviors, in particular voluntary ethanol consumption. Ethanol consumption is a more complex behavior than ethanol intoxication or tolerance, as it is not simply a physiological response but requires an animal to make a choice. Furthermore, the genes that influence ethanol intoxication and ethanol consumption are likely to be overlapping, but not identical. We thus sought to establish a paradigm for ethanol consumption in flies that would model at least some of the key features of alcohol addiction.
In our study, we used a two-choice feeding assay similar to the two-bottle choice assay used in rodent studies of ethanol consumption. Liquid food was presented in capillary tubes, and flies chose between food containing either 0% or 15% ethanol. Flies exhibited a robust, dose-dependent preference for the ethanol-containing food. While this preference was initially highly variable, ethanol preference was established by 24 hours and increased over a 5 day period. Voluntary ethanol consumption led to pharmacologically relevant ethanol concentrations in flies.
We characterized the sensory inputs that influence ethanol preference, and showed that while flies are attracted to the smell of ethanol, they are averse to its taste. However, olfactory attraction alone could not explain the preference for consuming ethanol, nor could attraction to the calories present in ethanol. These results suggested that flies might be attracted to ethanol as a drug, in addition to its role as a food source. To support this notion, we demonstrated that flies exhibit addiction-like behaviors when consuming ethanol. First, flies were willing to overcome an aversive stimulus, quinine, in order to consume ethanol. Second, flies rapidly returned to high levels of ethanol consumption after a 1 or 3 day period of ethanol deprivation, modeling a relapse-like effect.
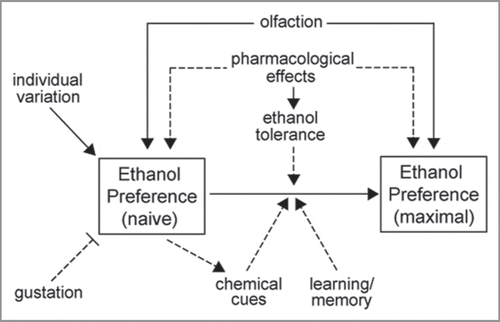
We speculate that ethanol preference in flies is likely to be influenced by a complex set of internal and external factors (). We have studied the roles of olfactory and gustatory inputs in influencing preference for ethanol and implicated the potential importance of its pharmacological effects. In addition to these factors, the increase in ethanol preference over time may reflect learning and memory processes that allow flies to reliably discriminate which capillaries contain the ethanol food. It is also possible that flies leave chemical cues such as aggregation pheromonesCitation19 to aid in this discrimination and reinforce their preference. The complexity of factors influencing ethanol preference may account for the initially high individual variation in this behavior.
Our study demonstrates that ethanol preference in Drosophila models characteristics of alcohol addiction. Like nearly all rodent models, this fly model does not encompass the entire spectrum of addiction behaviors. Our paradigm meets three of the six criteria classically proposed for an animal model of alcoholismCitation20 (voluntary consumption, pharmacologically relevant ethanol levels, and consumption that is not dependent on caloric or sensory effects) as well as a more recently proposed criterionCitation21 (relapse), but we have not yet demonstrated the other three criteria (tolerance, willingness to “work” for ethanol, and withdrawal symptoms when ethanol is removed). Future work may shed light on whether these phenomena are also associated with ethanol consumption in flies. Regardless, with this model in hand, the molecular and neural mechanisms underlying addiction-like behaviors in flies can now be investigated.
To begin to investigate the molecular pathways that influence ethanol preference, we identified one mutant, krasavietz (kra, also known as exba/eIF5c), that had decreased ethanol preference. kra also exhibited decreased ethanol sensitivity and strong defects in ethanol tolerance, suggesting that ethanol sensitivity and/or tolerance may influence ethanol preference. Kra is an evolutionarily conserved predicted translation initiation factor that inhibits translation in vitro.Citation22 Kra has been shown to interact with Short stop (Shot), a cytoskeletal crosslinking protein, to regulate filopodia formationCitation23 and midline axon guidance.Citation22 Both protein synthesis and cytoskeletal dynamics play important roles in cellular plasticity underlying drug addiction,Citation24–Citation26 and abused drugs have been reported to alter the expression of axon guidance molecules in the adult rodent brain,Citation27 making kra a tempting focus of study.
In addition to studying kra, unbiased genetic screens can identify novel genes that influence ethanol preference, which can then be tested in mammalian models. It will also be interesting to identify the neural circuitry underlying ethanol preference in flies and to determine whether it overlaps with the circuits known to mediate feeding (such as the hugin neuronsCitation28) or reward (such as the dopamineCitation29 and octopamine systemsCitation30). The availability of tools in Drosophila for manipulating both genes and neural circuits opens the door for studying the genetic and neural pathways involved in drug reward.
Figures and Tables
Figure 1 Potential factors influencing ethanol preference in flies and its increase over time. Solid lines indicate relationships supported by data; dashed lines indicate speculation. Preference for ethanol is likely influenced by its sensory and pharmacological properties. The increase in preference over time may depend on the development of ethanol tolerance as well as improved discrimination between food types, which could occur by learning or chemical cues deposited by flies.
Addendum to:
References
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry 2004; 61:807 - 816
- Dick DM, Foroud T. Candidate genes for alcohol dependence: a review of genetic evidence from human studies. Alcohol Clin Exp Res 2003; 27:868 - 879
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 1994; Edition 4 Washington DC American Psychiatric Association,
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol?. Alcohol 2008; 42:1 - 11
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet 2002; 32:363 - 388
- Koob GF. A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol 2004; 68:1515 - 1525
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol 2006; 11:195 - 269
- Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int Rev Neurobiol 2003; 54:199 - 228
- Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron 2000; 28:261 - 271
- Berger KH, Heberlein U, Moore MS. Rapid and chronic: two distinct forms of ethanol tolerance in Drosophila. Alcohol Clin Exp Res 2004; 28:1469 - 1480
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell 1998; 93:997 - 1007
- Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci USA 2005; 102:2141 - 2146
- Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett S, et al. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanolinduced behaviors. Cell 2009; 137:949 - 960
- Maas JW Jr, Vogt SK, Chan GC, Pineda VV, Storm DR, Muglia LJ. Calcium-stimulated adenylyl cyclases are critical modulators of neuronal ethanol sensitivity. J Neurosci 2005; 25:118 - 126
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature 1998; 396:366 - 369
- Richmond RC, Gerking JL. Oviposition site preference in Drosophila. Behav Genet 1979; 9:233 - 241
- Parsons PA. Larval responses to environmental ethanol in Drosophila melanogaster: variation within and among populations. Behav Genet 1980; 10:183 - 190
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA 2007; 104:8253 - 8256
- Bartelt RJ, Schaner AM, Jackson LL. cis-vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol 1985; 11:1747 - 1756
- Cicero TJ. Majchrowicz E, Noble EP. A critique of animal analogues of alcoholism. Biochemistry and Pharmacology of Ethanol 1979; 2:New York Plenum Press, 533 - 560
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol 1998; 12:339 - 369
- Lee S, Nahm M, Lee M, Kwon M, Kim E, Zadeh AD, et al. The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development 2007; 134:1767 - 1777
- Sanchez-Soriano N, Travis M, Dajas-Bailador F, Goncalves-Pimental C, Whitmarsh AJ, Prokop A. Mouse ACF7 and drosophila short stop modulate filopodia formation and microtubule organisation during neuronal growth. J Cell Sci 2009; 122:2534 - 2542
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 2004; 44:59 - 73
- Bramham CR. Local protein synthesis, actin dynamics and LTP consolidation. Curr Opin Neurobiol 2008; 18:524 - 531
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 2007; 8:844 - 858
- Bahi A, Dreyer JL. Cocaine-induced expression changes of axon guidance molecules in the adult rat brain. Mol Cell Neurosci 2005; 28:275 - 291
- Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol 2005; 3:1618 - 1629
- Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci 2007; 27:7640 - 7647
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 2003; 23:10495 - 10502