Abstract
We recently published a paper titled “Energetic Basis of Colonial Living in Social Insects” showing that basic features of whole colony physiology and life history follow virtually the same size-dependencies as unitary organisms when a colony’s mass is the summed mass of individuals. We now suggest that these results are evidence, not only for the superorganism hypothesis, but also for colony level selection. In addition, we further examine the implications of these results for the metabolism and lifetime reproductive success of eusocial insect colonies. We conclude by discussing the mechanisms which may underlie the observed mass-dependence of survival, growth and reproduction in these colonies.
Social insects occupy a central front in the long-standing battle regarding units of selection in the evolution of biological diversity.Citation1–Citation6 Ever since W.M. WheelerCitation7 coined the term “superorganism” there has been an historic dialectic among two camps over the ability for populations of eusocial insects to evolve based on adaptations, not of individual workers, but of the colonies in which they are a part. To skeptics the simplest explanation remains that selection acts solely on the inclusive fitness of a colony’s many individuals (or their genes).Citation5,Citation6,Citation8 In the other camp are those that posit that “superorganism” is more than a heuristic metaphor (e.g., queens are the colony’s ovaries and workers its soma) but that colonies indeed are organisms comprised of organisms that undergo selection as a single entity at a level above that of gene and individual.Citation1,Citation2,Citation7,Citation9,Citation10 While there remain calls for synthesis, based on bridging selfish genes with self-organization, 11 there also exists a need for predictions allowing one to differentiate colonies structured by relatedness versus those structured by ergonomic efficiency.Citation12
Here we suggest that one way to test the utility of the superorganism hypothesis is to test its predictions about the size scaling of life history traits. A basic prediction of the “gene-individual selection hypothesis” is that the reproductive behavior, growth rate, indeed all life history traits of a colony should arise from the summed behavior of a colony’s genes and individuals. For example, as long as an individual’s optimal metabolic rate fails to vary with colony mass this hypothesis predicts isometric scaling of colony metabolism. [We are unaware of any theory that predicts how tactics maximizing individual fitness in a eusocial insect colony should vary with colony size, and thus ultimately result in colony level metabolic allometries like those we discuss here].
In contrast, the “superorganism hypothesis” posits that selection acts on eusocial colonies just as it does on unitary organisms.Citation1 If so, the same ecological challenges should generate convergent phenotypic solutions regardless of the level of selection. Consider, for example, colonies of leaf cutter ants competing with cattle for the grass of an Argentine savannah. Both populations would tend to accumulate organisms effective at consuming grass, maintaining their microbial symbionts, and producing viable offspring. Likewise, the savannah’s 100 or so social insect species and innumerable unitary animal species face the same evolutionary challenge—consume biomass and convert it into efficiently into growth and reproduction. If there are a limited number of effective solutions set by physics, chemistry and evolutionary history, one would expect that the mass- specific allometries of growth and reproduction—the basic patterns of metabolic ecology— would converge, regardless of whether the organism was a colony or quadruped.
In our recently published paper titled “Energetic Basis of Colonial Living in Social Insects”,Citation13 we show that basic features of whole colony physiology and life history follow virtually the same sizedependencies as unitary organisms when a colony’s mass is the summed mass of individuals. Given the above reasoning, and in the absence of any similar theory arising from individual level selection, we suggest that this is evidence for the superorganism hypothesis, and hence colony level selection. Here we further develop the implications of these findings by making three points related to the size dependence of whole-colony metabolic rate, growth rate, reproductive rate and lifespan.
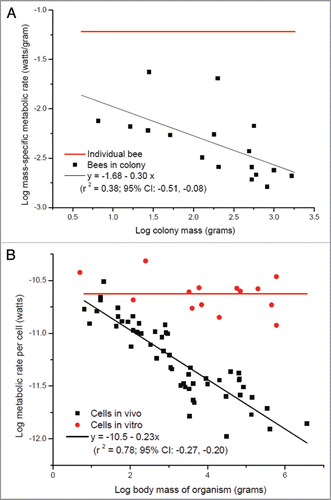
First, in Hou et al. 2010 we observed that whole colony metabolic rate scales sublinearly with whole colony mass where M is mass. Thus, whole colony metabolic rate is not simply the sum of individual metabolic rates but is proportional to about M0.75, similar to the scaling found in unitary insects. This allometry implies that the per-capita metabolic rate of an individual worker is independent of colony mass when outside the colony, but that on average, metabolic rates of workers in a colony decrease with increasing colony mass c. M−1/4. Here we provide some support for this hypothesis by showing that this appears to be the case in at least one species, the honey bee (). Note that this observation is remarkably similar to the metabolic rates of cells in a mammal: in vivo they scale to the −1/4 power of organism mass; in vitro and separated from the organism from which they derive, cellular metabolic rate is independent of organism mass ().
Next we showed that egg production rates also scaled as approximately M3/4, again mirroring the allometry of unitary organisms. Put another way, egg production is a constant fraction of the colony’s overall energy budget. One implication, consistent with the superorganism hypothesis, is that the metabolic rate of a colony governs the metabolic rate and egg production rate of its queen. As such, a queen’s often prolific egg productionCitation13 is only an outlier in the diversity of life when the queen is viewed as an individual.
Finally, we showed in Hou et al. 2010 that colony lifespan, as approximated by queen lifespan, scales with colony mass in the same way as lifespan scales with body mass in unitary insects (i.e., ∼M0.25). Again, the famously exceptional nature of social insect biology, this time the lifespans of queens,Citation14–Citation16 may disappear in the context of the superorganism hypothesis which views queens as the ovaries of a larger organism. Furthermore, these results present interesting questions regarding the relationship between colony metabolism, queen metabolism, and the lifespan of queens/colonies, because they suggest queen lifespan is inversely proportional to mass-specific colony metabolic rate. On the one hand, from the superorganism perspective, these results suggests that so long as queens are not considered the “organism”, Pearl’s rate of living hypothesis applies to queens as well as it does to unitary organisms. That is, in superorganisms as in unitary organisms, lifespan in inversely proportional to the rate of living (i.e., mass-specific metabolic rate).Citation17 On the other hand, from the perspective of the queen as an individual, it is difficult to see how queen metabolic rate and queen lifespan can be inversely related. In either case, the observations about the scaling of queen lifespan with colony mass (i.e., M1/4), combined with the observation that mass-specific biomass production is inversely proportional to colony lifespan (i.e., M−1/4), implies that the lifetime reproductive success of colonies is approximately invariant with respect to colony size, as is generally the case for unitary organisms (i.e., M1/4 × M−1/4 = M0).
The patterns we reveal, using metabolic theory, provide a point of departure for the study of the sociality, physiology and life history of whole colonies and the individuals comprising those colonies. They reveal a need for mechanisms underlying the flux, storage, and turnover of energy in these groups. Many factors may contribute to the sub-linear scaling because as colony sizes increase (ontogenetically and/or comparatively across species), so frequently does worker size,Citation18–Citation21 polymorphismCitation22–Citation24 and the division of labor.Citation25,Citation26 Each may lead to a decrease of per-capita energy consumption of individuals (reviewed in ref. Citation27); each may thus contribute to sub-linear metabolic scaling.
Other candidates exist. Nest architecture, variable with age and across species (reviewed in ref. Citation28), may constrain metabolism in a variety of ways including gas exchange. Similarly, gas/heat exchange may scale allometrically if the way individuals “clump” in the nest varies with colony size, in turn shaping the colony’s collective surface area to volume ratio. Finally, at least one model has shown that fractal-like foraging trails of some ants can generate something close to the observed scaling of colony metabolic rateCitation29 under the assumption that selection is acting on whole colonies. These mechanisms, their potential “dopplegangers” in the structure and function of unitary organisms, and their implications for selection at the colony level, are ripe for exploration given the remarkable congruences revealed by metabolic theory.
Figures and Tables
Figure 1 (A) Mass-specific metabolic rate of honey bees as function of colony mass (data of colonies from;Citation13 data of individual bee is averaged from ref. 30 and 31). (B) Metabolic rate of single mammalian cells as function of body mass (reviewed in refs. 32 and 33).
Acknowledgements
Sean O’Donnell and Jon Shik provided many useful comments on this manuscript.
Addendum to:
References
- Wilson DS, Sober E. Reviving the superorganism. J Theor Biol 1989; 136:337 - 356
- Hölldobler B, Wilson EO. The Superorganism: The beauty, elegance and strangeness of insect societies 2008; New York W.W. Norton & Company,
- Odum EP. The strategy of ecosystem development. Science 1969; 164:262 - 270
- Wynne-Edwards VC. Evolution through group selection 1986; Oxford Blackwell,
- West-Eberhard MJ. Temporary queens in Matapolybia wasps: non-reproductive helpers without altruism?. Science 1978; 200:441 - 443
- Williams GC. Dawkins R, Ridley M. A defense of reductionism in evolutionary biology. Oxford surveys in evolutionary biology 1986; Oxford Oxford University Press, 1 - 27
- Wheeler WM. The ant-colony as an organism. J morphol 1911; 22:307 - 325
- Foster KR, Wenseleers T, Ratnieks FLW. Kin selection is the key to altruism. Trends Ecol Evol 2006; 21:57 - 60
- Emerson AE. Social coordination and the superorganism. Am Midl Nat 1939; 21:182 - 209
- Seeley TD. The Wisdom of the hive: The social physiology of honey bee colonies 1996; Cambridge MA Harvard University Press,
- Boomsma JJ, Franks NR. Social insects: from selfish genes to self organisation and beyond. Trends Ecol Evol 2006; 21:303 - 308
- Hammond RL, Keller L. Conflict over male parentage in social insects. PLoS Biol 2004; 2:1472 - 1482
- Hou C, Kaspari M, Vander Zanden HB, Gillooly JF. Energetic basis of colonial living in social insects. Proc Natl Acad Sci USA 2010; 107:3634 - 8363
- Jemielity S, Chapuisat M, Parker JD, Keller L. Long live the queen: studying aging in social insects. Age 2005; 27:241 - 248
- Keller L. Queen lifespan and colony characteristics in ants and termites. Insectes Soc 1998; 45:235 - 246
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature 1997; 389:958 - 960
- McCoy MW, Gillooly JF. Predicting natural mortality rates of plants and animals. Ecol Lett 2008; 11:710 - 716
- Tschinkel WR. Sociometry and sociogenesis of colonies of the harvester ant, Pogonomyrmex badius: worker characteristics in relation to colony size and seasons. Insectes Soc 1998; 45:385 - 410
- Brian MV, Elmes GW. Production by ant Tetramorium caespitum in a Southern english heath. J Anim Ecol 1974; 43:889 - 903
- Tschinkel WR. Sociometry and sociogenesis of colonies of the fire ant Solenopsis invicta during one annual cycle. Ecol Monogr 1993; 63:425 - 457
- Nowbahari E, Feneron R, Malherbe MC. Polymorphism and polyethism in the formicinae ant Cataglyphis niger (Hymenoptera). Sociobiology 2000; 36:485 - 496
- Wetterer JK. The ecology and evolution of worker size-distribution in leaf-cutting ants (Hymenoptera: Formicidae). Sociobiology 1999; 34:119 - 144
- Wilson EO. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). Behav Ecol Sociobiol 1983; 14:55 - 60
- Beshers SN, Traniello JFA. The adaptiveness of worker demography in the attine ant Trachymymrex septentrionalis. Ecology 1994; 75:763 - 775
- Wilson EO. The Insect Societies 1971; Cambridge MA Belknap Press of Harvard Univ. Press,
- Hölldobler B, Wilson EO. The ants 1990; Cambridge MA Belknap Press of Harvard Univ. Press,
- Chown SL, Marais E, Terblanche JS, Klok CJ, Lighton JRB, Blackburn TM. Scaling of insect metabolic rate is inconsistent with the nutrient supply network model. Funct Ecol 2007; 21:282 - 290
- Tschinkel WR. The nest architecture of the ant, Camponotus socius. J Insect Sci 2005; 5:9
- Jun J, Pepper JW, Savage VM, Gillooly JF, Brown JH. Allometric scaling of ant foraging trail networks. Evol Ecol Res 2003; 5:297 - 303
- Stabentheiner A, Vollmann J, Kovac H, Crailsheim K. Oxygen consumption and body temperature of active and resting honeybees. J insect physiol 2003; 49:881 - 889
- Withers PC. The effects of ambient air pressure on oxygen consumption of resting and hovering honeybees. J Comp Physiol B 1981; 141:433 - 437
- West GB, Woodruff WH, Brown JH. Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc Natl Acad Sci USA 2002; 99:2473 - 2478
- Brown MF, Gratton TP, Stuart JA. Metabolic rate does not scale with body mass in cultured mammalian cells. Am J Physiol-Reg I 2007; 292:2115 - 2121