Abstract
The host transmembrane protein BST-2/tetherin is a powerful antiviral factor that blocks the production of enveloped viruses. The HIV-1 accessory protein Vpu inhibits the antiviral activity of BST-2; however, the degradation pathway by which Vpu downregulates BST-2 from the cell surface and the actual subcellular location where Vpu targets BST-2 for downregulation remain controversial. Whereas one study showed that Vpu acts on constitutively endocytosed BST-2, we recently reported that Vpu can internalize BST-2 from the cell surface. Because the evidence for this conclusion was derived from indirect results, we present direct evidence in this study using an antibody internalization assay with an endocytosis-defective mutant of BST-2. The internalization of the BST-2 protein into cells coexpressing wild-type Vpu was observed when the cells were preincubated with antibodies against BST-2 at 37˚C, but not at 4˚C, for 10 min. These results strongly support our previous finding that continuously expressed de novo BST-2 at the cell surface is internalized by functional Vpu protein.
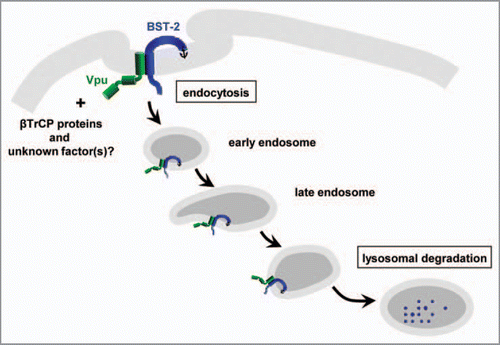
The HIV-1 accessory protein Vpu plays two distinct roles in virus replication: (1) the downmodulation of the HIV-1 receptor CD4 in the endoplasmic reticulum (ER);Citation1,Citation2 and (2) the inhibition of BST-2/tetherin (referred to hereafter as BST-2) function, which was recently identified as a powerful blocker of HIV-1 virion production.Citation3,Citation4 The former function leads to the proteasomal degradation of CD4,Citation5–Citation7 which requires a subunit of the Skp1-Cullin1-F-box ubiquitin ligase complex, β-transducin repeat-containing protein 1 (βTrCP-1),Citation6 and βTrCP-2.Citation8 The latter function of Vpu results in the lysosomal degradationCitation9–Citation11 (or in the proteasomal degradationCitation12,Citation13) of BST-2 through transmembrane (TM) interactions between Vpu and BST-2,Citation10,Citation13–Citation16 and is dependent on βTrCP proteins.Citation9–Citation12
Our recent observations showed that Vpu actively induces the internalization of BST-2 from the cell surface,Citation10 whereas another study showed that the subcellular targeting of BST-2 by Vpu is post-endocytic.Citation11 Still, the evidence we presented was indirect for the following reasons: (1) the study utilized a dynamin-2 dominantnegative protein for inhibition of both clathrin-dependent and -independent, but not caveolae/lipid raft-dependent endocytosis,Citation17 and revealed that this inhibition rescued BST-2 expression downregulated by Vpu; (2) the BST-2 mutant deficient in constitutive endocytosis (Y6A/Y8A) remained sensitive to Vpu with respect to the level of BST-2 cell-surface expression and inhibition of virion production.
This study uses an antibody internalization assay to directly demonstrate that BST-2, which is downregulated by Vpu and degraded in lysosomes, originates from the plasma membrane. This experiment utilized the BST-2 Y6A/Y8A mutant to avoid antibody internalization by physiological endocytosis of BST-2. COS7 cells were co-transfected with a plasmid expressing EGFP-fused wildtype (WT) or mutant Vpu (CD4tmVpu; Vpu carrying TM domain of CD4 that is unable to bind the TM of BST-2Citation10) carrying the Rev-responsive element, together with the Rev expression plasmid, and an extracellular Myc-tagged BST-2 Y6A/Y8A mutant plasmid. After cultivation for 24 h, the transfected cells were then preincubated in complete medium with anti-Myc mouse monoclonal antibodies at 37°C for 10 min (or 4°C for 10 min). The cells were washed in PBS at 4°C and then fixed with 4% paraformaldehyde either immediately or after an additional incubation in complete medium in the presence of lysosomal protease inhibitors (leupeptin and pepstatin A). The fixed cells were permeabilized with 0.05% saponin and immunostained with polyclonal antibodies against a lysosome marker, cathepsin D and Cy3-conjugated goat anti-mouse and Cy5-conjugated goat anti-rabbit secondary antibodies. Nuclear staining was performed with Hoechst. All immunofluorescence images were captured as described previously.Citation10
Originally, we performed internalization experiments at 4°C for 10 min followed by incubation at 37°C for an additional 80 min. Under these conditions, however, we were unable to detect any signal for BST-2 in the cells coexpressing WT Vpu (, left), while in the cells coexpressing the mutant Vpu, robust signals for BST-2 were detected (, right). The results were completely consistent with our recent data from flow cytometry analysis.Citation10 Based on these results, we hypothesized that preincubation of the cells with antibodies at 37°C (instead of 4°C) for 10 min might enable the visualization of undetectable levels of BST-2 by capturing the continuous de novo cell-surface expression of the protein.
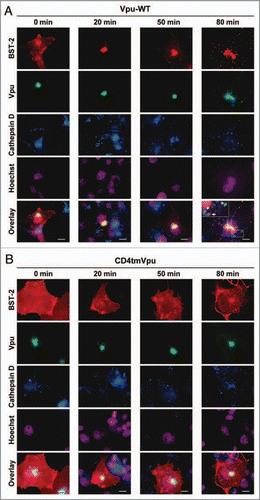
To examine this possibility, we preincubated the cells with antibodies at 37°C for 10 min, and after an additional incubation, stained the cells with the secondary antibodies. After the 10 min preincubation (0 min), BST-2 signals were clearly detected in the cells expressing WT Vpu (, first column) as expected. After 20 and 50 min, the signals accumulated at the perinuclear region (, second and third columns) and then were dispersed into the cytoplasmic compartments after 80 min (, fourth column). Importantly, a magnified image of a selected area revealed the clear colocalization of BST-2 with Vpu and cathepsin D (, fourth column, square in the lowest panel). On the other hand, BST-2 signals remained at the surface of the cells expressing the mutant Vpu protein throughout the observations (). It should be noted that non-specific signals for BST-2 were not detected in cells that did not express BST-2, suggesting that the antibodies were not non-specifically internalized into the cells. Together, these data present strong evidence for the direct internalization of cell-surface BST-2 by Vpu.
In conclusion, this study confirms that Vpu does not wait below the plasma membrane for cell-surface BST-2 to be constitutively endocytosed, but actively and quickly internalizes this host antiviral factor through TM-to-TM interactions, leading to lysosomal degradation, as depicted in . Further understanding the mechanisms by which Vpu blocks BST-2 (e.g., the identification of unknown Vpu-binding partners other than βTrCP proteins) may provide new insights into potential therapeutic strategies targeting Vpu.
Figures and Tables
Figure 1 Antibody internalization assay (with preincubation at 4°C for 10 min). COS7 cells were co-transfected as described previously,Citation10 with pCA-Vpu-EGFP-RRE (WT or CD4tm mutant), pCA-Rev, and Myc-tagged BST-2 (pCA-BST-2-exMyc-Y6A/Y8A) and cultured for 24 h. To detect Myc-tagged BST-2 internalized at the plasma membrane, transfected cells were cultured in complete medium in the presence of anti-Myc mouse monoclonal antibodies (1:50 dilution) at 4°C for 10 min. After an additional 80 min in complete medium in the presence of lysosomal protease inhibitors (40 μM of leupeptin and pepstatin A), the cells were washed in PBS at 4°C and then fixed with 4% paraformaldehyde. The fixed cells were permeabilized with 0.05% saponin and immunostained with rabbit anti-cathepsin D (1:200). Cy3-conjugated goat anti-mouse and Cy5-conjugated goat anti-rabbit secondary antibodies were used at 5 μg/ml. DN A staining with Hoechst was performed at 0.5 μg/ml. All immunofluorescence images were captured as described previously.Citation10 Bars, 10 μm.
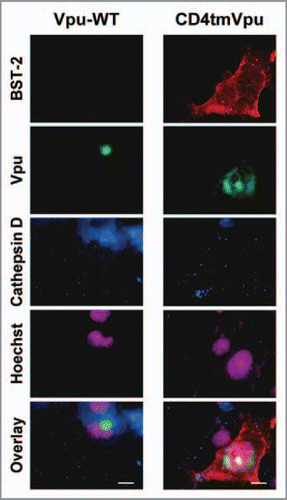
Figure 2 Antibody internalization assay (with preincubation at 37°C for 10 min). Same as in , but the cells transfected with the WT (A) or mutant (B) Vpu expression plasmid were cultured in complete medium with anti-Myc mouse monoclonal antibodies at 37°C instead of 4°C for 10 min, and then fixed with 4% paraformaldehyde either immediately (0 min) or after an additional incubation (20, 50 or 80 min). Squares outline magnified regions where the colocalization of BST-2, Vpu, and a lysosome marker cathepsin D is indicated by arrows. Bars, 10 µm.
Acknowledgements
This work was supported by a grant from the Ministry of Health, Labor and Welfare of Japan.
Addendum to:
References
- Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol 1992; 66:7193 - 7200
- Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J Virol 1992; 66:226 - 234
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008; 451:425 - 430
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, et al. The interferoninduced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 2008; 3:245 - 252
- Fujita K, Omura S, Silver J. Rapid degradation of CD4 in cells expressing human immunodeficiency virus type 1 Env and Vpu is blocked by proteasome inhibitors. J Gen Virol 1997; 78:619 - 625
- Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, et al. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell 1998; 1:565 - 574
- Schubert U, Anton LC, Bacik I, Cox JH, Bour S, Bennink JR, et al. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J Virol 1998; 72:2280 - 2288
- Butticaz C, Michielin O, Wyniger J, Telenti A, Rothenberger S. Silencing of both beta-TrCP1 and HOS (beta-TrCP2) is required to suppress human immunodeficiency virus type 1 Vpu-mediated CD4 down-modulation. J Virol 2007; 81:1502 - 1505
- Douglas JL, Viswanathan K, McCarroll MN, Gustin JK, Fruh K, Moses AV. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism. J Virol 2009; 83:7931 - 7947
- Iwabu Y, Fujita H, Kinomoto M, Kaneko K, Ishizaka Y, Tanaka Y, et al. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J Biol Chem 2009; 284:35060 - 35072
- Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, et al. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via β-TrCP and endo-lysosomal trafficking. PLoS Pathog 2009; 5:1000450
- Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog 2009; 5:1000574
- Goffinet C, Allespach I, Homann S, Tervo H-M, Habermann A, Rupp D, et al. HIV-1 Antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 2009; 5:285 - 297
- Gupta RK, Hue S, Schaller T, Verschoor E, Pillay D, Towers GJ. Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog 2009; 5:1000443
- McNatt MW, Zang T, Hatziioannou T, Bartlett M, Fofana IB, Johnson WE, et al. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog 2009; 5:1000300
- Rong L, Zhang J, Lu J, Pan Q, Lorgeoux RP, Aloysius C, et al. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. J Virol 2009; 83:7536 - 7546
- Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci 2003; 116:4707 - 4714