Abstract
Despite its fundamental role in centrosome biology, procentriole formation both in the canonical and in the de novo replication pathways remains poorly understood, and the molecular components that are involved in human cells are not well established. We found that one of the tubulin cofactors, TBCD, is localized at centrosomes and the midbody, and is required for spindle organization, cell abscission, centriole formation, and ciliogenesis. Our studies have established a molecular link between the centriole and the midbody, demonstrating that this cofactor is also necessary for microtubule retraction during cell abscission. TBCD is the first centriolar protein identified that plays a role in the assembly of both “centriolar rosettes” during early ciliogenesis, and at the procentriole budding site by S/G2, a discovery that directly implicates tubulin cofactors in the cell division, cell migration, and cell signaling research fields.
Microtubules are alternating αβ-tubulin polymers where each α-tubulin subunit interacts with two β-tubulin molecules along the protofilament and two other α-tubulins at either side, forming the αβ-tubulin heterodimer and actual microtubule subunit of the polymer.Citation1 In this way, the acquisition of the quaternary structure by tubulins is an intricate process where a huge number of protein-protein interactions have to be precisely controlled in order to direct one molecule of α-tubulin to bind a single β-tubulin subunit in the correct position.
The discovery of the so-called “postchaperonin tubulin folding pathway”,Citation2 and the subsequent identification of the tubulin cofactors involved in this process, have driven much of the effort in the study of the implications of these proteins in the folding/dimerization pathway.Citation3–Citation6 However, more recent results have shown that tubulin cofactors also participate in the proteostasis of the tubulin dimer through their intrinsic ability to dissociate α- from β-tubulin. Given that the tubulin dissociation constant is close to 10−11 M,Citation7 it is quite reasonable to postulate that these proteins must also be required in tubulin proteostasis.Citation8 Moreover, their ability to dissociate the tubulin heterodimer in a controlled way is a mechanism that certain types of cells exploit to regulate key cytoskeletal processes, such as controlling their microtubule densities in macrophagesCitation9 or the trimming of the distal microtubule tips at the axonal growth cone terminal in neurons.Citation10
Regarding the possible roles of tubulin cofactors at the centrosome, it seems reasonable to assume that tubulin cofactors must operate here as a machinery to associate or dissociate tubulin for microtubule polymerization or depolymerization, because microtubule organizing centers (MTOCs), and in particular the centrosome, accumulate αβ-tubulin polypeptides for microtubule nucleation.Citation11 Although our work demonstrates that TBCD is a centriolar protein implicated in the actual process of centriologenesis that concentrates around the proximal end of the mother centriole during centriole biogenesis and during the cell cycle, we also found that TBCD is implicated in the assembly and maintenance of the bipolar mitotic spindle, such as for many centriolar-centrosomal proteins. Furthermore, TBCD localizes at the Fleming bodies during the final steps of telophase where it participates in microtubule retraction during cytokinesis and cell abscission.Citation12 Summarizing, these results make it obvious how tubulin cofactors, and in particular TBCD, being key participants in cell division cytoskeletal reorganization, are more than tubulin folding proteins.
Centriologenesis also occurs in other systems such as in multiciliated cells where masses of centrioles are assembled during their differentiation process.Citation13,Citation14 While the canonical centriole duplication occurs once per cycle and it is tightly controlled and coordinated with chromosomal duplication events, in these cells centriolar amplification is achieved by a mechanism whereby several daughter centrioles are simultaneously nucleated from a mother centriole, or alternatively noncentriolar structures named “deuterosomes.”Citation15 Our work also investigates the implication of TBCD in this process by developing a new system model that allows the study of cilial development in primary cultures from mammalian ependymal cells. As anticipated from the data indicated above, we show that TBCD also participates in basal body assembly in differentiating ciliated cells where there is a recruitment of this cofactor into round structures, of approximately 0.3 µm in diameter surrounding a γ-tubulin central spot, which are called “centriolar rosettes.” These highly organized structures () have so far only been described under physiological conditions by qualitative electron microscopy, and have seldom been observed when the SAK/PLK4 kinase is overexpressed, or in cells artificially arrested at the S phase.Citation16 Thus to date, TBCD is the first ever reported protein to be recruited in these procentriolar formations.Citation17 Further biochemical studies will shed light on the mechanisms underlying the way this tubulin cofactor is recruited during procentriolar assembly in differentiating multiciliated cells. Furthermore, preliminary studies on flagellated cells have also shown that TBCD is detectable at the base of the mammalian spermatozoa flagellum (), thus providing further evidence of the role of TBCD in flagellogenesis where, as is the case of cilia, highly sophisticated microtubules are also assembled and maintained. The question to answer now is what TBCD does at the centriole and basal bodies. Although the mechanisms involved in centriolar and ciliary assembly are still obscure, it is certain that centriologenesis and ciliogenesis (or flagellogenesis) require, above all, a great amount of tubulin supply and tubulin processing, and these two events are directly associated with the tubulin cofactors.
In view of the fact that cilia and flagella are ancient evolutionarily conserved organelles whose importance has been increasingly recognized during this decade, findings describing key processes involved in their assembly, disassembly and maintenance, as well as their biological roles are crucial to understand the broad series of pathologies caused by defects in cilia termed “ciliopathies.”Citation18 Ciliopathies result in a plethora of defective biological processes including alterations in the movement of fluids, cell locomotion, sexual reproductive roles, left-right axis pattern formation, neural patterning, cerebrospinal fluid flow, mucociliary clearance and, more recently, in cellular signaling and cell environmental sensing. These ciliary implications have opened up an enormous biomedical research field aimed at understanding a wide range of human disorders where cilia and flagella are directly and indirectly implicated: from infertility, to more complex syndromes including Bardet-Biedl syndrome, Alstrom syndrome, Meckel-Gruber syndrome, retinal degeneration, polycystic kidney diseases and neural tube defects. Moreover, since cilia have receptors for signaling molecules including Sonic hedgehog and platelet-derived growth factor (Shh and PDGF),Citation19,Citation20 new lines of evidence suggest that the frequent deregulation of these pathways during cell transformation, together with the common disappearance of cilia in transformed cells, raises the possibility that defective ciliary signaling may promote cancer.Citation21
Figures and Tables
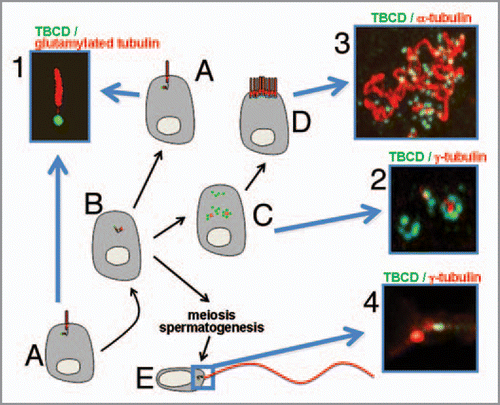
Figure 1 (A) Typical cell in G0/G1 exhibiting a primary, also known as a solitary, cilium. (1) These structures,Citation22–Citation24 which typically label with antibodies recognizing glutamylated tubulin (red), arise from a basal body that, as this image shows, contains abundant TBCD (in green). (B) Cells, both undergoing mitosis and differentiating into multiciliated cells, must assemble new centrioles. (C) Cells differentiating into multiciliated cells (such as ependymal cells) disassemble their primary cilium prior to undergoing massive procentriole assembly. This phenomenon is achieved by a mechanism whereby several daughter procentrioles containing abundant TBCD (2, green) are simultaneously nucleated from a mother centriole containing γ-tubulin (2, in red). (D) Assembled centrioles next migrate to the apical cell surface where they become basal bodies of cilia (3). As is the case for a primary cilium, a single TBCD structure (green) is also situated at the base of each motile cilium. (E) TBCD is also localized at the centrioles of the base of the flagellum in human spermatozoa (4).
Acknowledgements
This work was supported by grants from the Consolider-Ingenio Spanish Ministry of Education and Science Centrosome-3D and BFU2007-64882 and the IFIMAV. We also thank to Laura Alvarez, Begoña Ubilla and Isabel Rodriguez for technical help.
References
- Li H, DeRosier DJ, Nicholson WV, Nogales E, Downing KH. Microtubule structure at 8 A resolution. Structure 2002; 10:1317 - 1328
- Fontalba A, Paciucci R, Avila J, Zabala JC. Incorporation of tubulin subunits into dimers requires GTP hydrolysis. J Cell Sci 1993; 106:627 - 632
- Lewis SA, Tian G, Cowan NJ. The alpha- and beta-tubulin folding pathways. Trends Cell Biol 1997; 12:479 - 484
- López-Fanarraga M, Avila J, Guasch A, Coll M, Zabala JC. Review: postchaperonin tubulin folding cofactors and their role in microtubule dynamics. J Struct Biol 2001; 135:219 - 229
- Szymanski D. Tubulin folding cofactors: half a dozen for a dimer. Curr Biol 2002; 12:767 - 769
- Fanarraga ML, Abad X, Kortazar D, Bellido J, Villegas JC, Zabala JC. Structure and function of the mammalian tubuling folding cofactors. Recent Res Dev Biochem 2003; 4:575 - 587
- Caplow M, Fee L. Dissociation of the tubulin dimer is extremely slow, thermodynamically very unfavorable, and reversible in the absence of an energy source. Mol Biol Cell 2002; 13:2120 - 2131
- Keller CE, Lauring BP. Possible regulation of microtubules through destabilization of tubulin. Trends Cell Biol 2005; 15:571 - 573
- Fanarraga ML, Villegas JC, Carranza G, Castaño R, Zabala JC. Tubulin cofactor B regulates microtubule densities during microglia transition to the reactive states. Exp Cell Res 2 2009; 315:535 - 541
- López-Fanarraga M, Carranza G, Bellido J, Kortazar D, Villegas JC, Zabala JC. Tubulin cofactor B plays a role in the neuronal growth cone. J Neurochem 2007; 100:1680 - 1687
- Lüders J, Stearns T. Microtubule-organizing centres: a re-evaluation. Nat Rev Mol Cell Biol 2007; 8:161 - 167
- Doxsey SJ. Molecular links between centrosome and midbody. Mol Cell 2005; 20:170 - 172
- Loots GP, Nel PP. Early stages of ciliogenesis in the respiratory epithelium of the nasal cavity of rabbit embryos. Cell Tissue Res 1989; 255:589 - 594
- Vladar EK, Stearns T. Molecular characterization of centriole assembly in ciliated epithelial cells. J Cell Biol 2007; 178:31 - 42
- Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol 2007; 8:451 - 463
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, et al. Plk4-induced centriole biogenesis in human cells. Dev Cell 2007; 13:190 - 202
- Strnad P, Gönczy P. Mechanisms of procentriole formation. Trends Cell Biol 2008; 18:389 - 396
- Nigg EA, Raff JW. Centrioles, centrosomes and cilia in health and disease. Cell 2009; 139:663 - 678
- Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH Jr, et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 2009; 15:1055 - 1061
- Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol 2009; 19:1498 - 1502
- Han YG, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol 2010; 20:58 - 67
- Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell 2007; 129:1255 - 1257
- Seeley ES, Nachury MV. The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci 2010; 123:511 - 518
- Santos N, Reiter JF. Building it up and taking it down: the regulation of vertebrate ciliogenesis. Dev Dyn 2008; 237:1972 - 1981