Abstract
The role of photosynthesis in plant defense is a fundamental question awaiting further molecular and physiological elucidation. To this end we investigated host responses to infection with the bacterial pathogen Xanthomonas axonopodis pv. citri, the pathogen responsible for citrus canker. This pathogen encodes a plant-like natriuretic peptide (XacPNP) that is expressed specifically during the infection process and prevents deterioration of the physiological condition of the infected tissue. Proteomic assays of citrus leaves infected with a XacPNP deletion mutant (∆XacPNP) resulted in a major reduction in photosynthetic proteins such as Rubisco, Rubisco activase and ATP synthase as a compared with infection with wild type bacteria. In contrast, infiltration of citrus leaves with recombinant XacPNP caused an increase in these host proteins and a concomitant increase in photosynthetic efficiency as measured by chlorophyll fluorescence assays. Reversion of the reduction in photosynthetic efficiency in citrus leaves infected with ∆XacPNP was achieved by the application of XacPNP or Citrus sinensis PNP lending support to a case of molecular mimicry. Finally, given that ∆XacPNP infection is less successful than infection with the wild type, it appears that reducing photosynthesis is an effective plant defense mechanism against biotrophic pathogens.
Natriuretic peptides (NPs) are hormones strongly implicated in the regulation of salt and water balance in vertebrates. In higher plants, the heterologous plant NPs (PNPs) elicit a number of responses that contribute to the regulation of homeostasis and growth.Citation1 PNPs act via rapid and transient increases in cellular cGMP levelsCitation2 and promote tissue specific ion movements,Citation3 increases in net water uptake into cells as well as stomatal opening.Citation4–Citation6 PNPs are upregulated under conditions of osmotic stressCitation7 and K+ starvationCitation8 and have been localized in conductive tissue.Citation9 Furthermore, PNPs have been identified in the apoplastic proteomeCitation10 and biologically active PNP was isolated from xylem exudates.Citation9
We found that the citrus canker causing bacteria Xanthomonas axonopodis pv. citri (Xac), but no other phytopathogen or bacteria, has a PNP-like gene (XacPNP)Citation11 and that this gene is expressed during Xac infection suggesting a role in pathogenicity.Citation12 We also observed that lesions produced in leaves infected with a XacPNP deletion mutant (ΔXacPNP) were more necrotic than those infected with the wild type and that the mutant causes the formation of highly necrotic tissue leading to earlier bacterial cell death.Citation12 We also demonstrated that recombinant XacPNP, much like PNPs, can cause plant responses such as stomatal opening as well as improved tissue hydration.Citation12 This would suggest that the plant-like bacterial PNP enables the plant pathogen to modify host responses thereby creating conditions favorable to its own survival.Citation12,Citation13
With a view to further characterize responses to PNP we undertook proteomics studies on citrus leaves infected with Xac and observed a decrease in the expression of sugar-regulated photosynthetic proteins such as Rubisco and Rubisco activase and also of ATP synthase and an increase in NADH dehydrogenase diagnostic for a reduction in photosynthetic efficiency during citrus canker ).Citation14 This indicates that during pathogen attack the biosynthesis of defense-related compounds are a priority for the plant while other (e.g., growth related) cellular activities are reduced thus permitting a reduction in photosynthetic rates until pathogenic growth has been halted.Citation15,Citation16 Such a reduction in photosynthesis may starve biotrophic pathogens of nutrients thereby benefitting the plant. Further, we analyzed the host proteome after infection with a XacPNP deletion mutant strain (ΔXacPNP) and observed that the main difference between infections with Xac wild type and ΔXacPNP was in proteins with a key role in carbon metabolism and photosynthesis showing that ΔXacPNP caused a considerably bigger reduction in the expression of photosynthesis genes ().Citation14 Consistent with this we observed that application of recombinant XacPNP to leaves increases the expression levels of these photosynthetic proteins ().Citation17
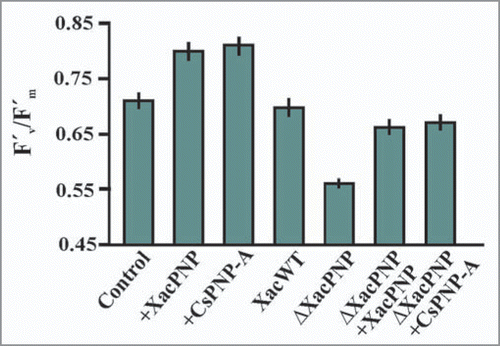
Since the XacPNP gene is only present in X. axonopodis pv. citri we consider it likely that this gene has been acquired by the bacteria in an ancient lateral gene transfer event and speculated that this might be a case of molecular mimicry where the pathogen modulates photosynthesis and consequently homeostasis to its own advantage. In order to assess this hypothesis we compared how XacPNP and its plant homolog Citrus sinensis PNP (CsPNP-A) modify photosynthetic performance by examining chlorophyll fluorescence parameters after 30 minutes of incubation in leaves infiltrated with purified peptides at a concentration of 5 µM. We noted that photosynthetic efficiency is enhanced in the presence of either purified peptides compared with control leaves (). Similar to our previous workCitation14 we observed a reduced photosynthetic efficiency in wild type infiltrations as compared to control leaves and a larger reduction in ΔXacPNP infiltrated tissue (). We also observed that the reduction in photosynthetic efficiency caused by ΔXacPNP can be complemented by co-infiltration with either recombinant XacPNP or CsPNP-A () hence adding further strength to the case of molecular mimicry. Given that keeping photosynthesis going is advantageous to the biotrophic pathogen, the converse implies that shutting photosynthesis down must be beneficial to the host and may be an important part of plant defense.
Figures and Tables
Figure 1 Changes in photosynthetic proteins during bacterial infections and XacPNP treatment. Protein spots from 2-DE SDS-PAGE of proteins from citrus leaves stained with Coomassie blue. Citrus leaves were infiltrated with XacWT, ΔXacPNP (107 CFU/ml) and 5 µM XacPNP pure protein (+XacPNP). After 3 days of bacterial infections or 30 minutes after infiltration with recombinant protein, total plant proteins were extracted and subjected to the proteomics analysis. As control, citrus leaves were infiltrated with Tris 50 mM.
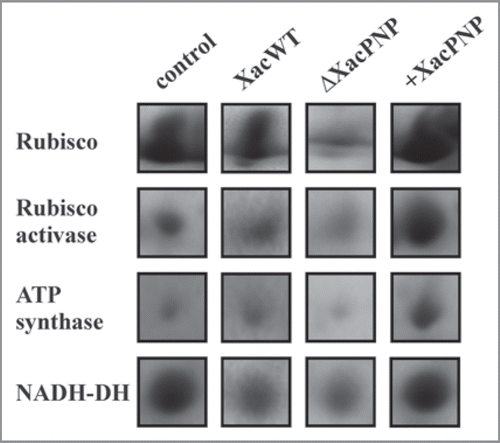
Figure 2 Effect of PNPs in effective quantum efficiency of photosystem II in host leaves. Chlorophyll fluorescence was measured by an 0.8 s saturating pulse at 5,000 mmol m−2 s−1 in leaves infiltrated with 5 µM PNPs, Xac wild type, ΔXacPNP and ΔXacPNP (107 CFU/ml) complemented with XacPNP and CsPNP-A. In the control, citrus leaves were infiltrated with Tris 50 mM. The results are the mean of three replicates and error bars represent the standard deviations.
Addendum to: and
References
- Gehring CA, Irving HR. Natriuretic peptides—a class of heterologous molecules in plants. Int J Biochem Cell Biol 2003; 35:1318 - 1322
- Pharmawati M, Billington T, Gehring CA. Stomatal guard cell responses to kinetin and natriuretic peptides are cGMP-dependent. Cell Mol Life Sci 1998; 54:272 - 276
- Ludidi N, Morse M, Sayed M, Wherrett T, Shabala S, Gehring C. A recombinant plant natriuretic peptide causes rapid and spatially differentiated K+, Na+ and H+ flux changes in Arabidopsis thaliana roots. Plant Cell Physiol 2004; 45:1093 - 1098
- Pharmawati M, Shabala SN, Newman IA, Gehring CA. Natriuretic peptides and cGMP modulate K+, Na+ and H+ fluxes in Zea mays roots. Mol Cell Biol Res Commun 1999; 2:53 - 57
- Maryani MM, Bradley G, Cahill DM, Gehring CA. Natriuretic peptides and immunoreactants modify the osmoticum-dependent volume changes in Solanum tuberosum L. mesophyll cell protoplasts. Plant Sci 2001; 161:443 - 452
- Morse M, Pironcheva G, Gehring C. AtPNP-A is a systemically mobile natriuretic peptide immunoanalogue with a role in Arabidopsis thaliana cell volume regulation. FEBS Lett 2004; 556:99 - 103
- Rafudeen S, Gxaba G, Makgoke G, Bradley G, Pironcheva G, Raitt L, et al. A role for plant natriuretic peptide immuno-analogues in NaCland drought-stress responses. Physiol Plant 2003; 119:554 - 562
- Meier S, Bastian R, Donaldson L, Murray S, Bajic V, Gehring C. Co-expression and promoter content analyses assign a role in biotic and abiotic stress responses to plant natriuretic peptides. BMC Plant Biol 2008; 8:24
- Maryani MM, Morse MV, Bradley G, Irving HR, Cahill DM, Gehring CA. In situ localization associates biologically active plant natriuretic peptide immuno-analogues with conductive tissue and stomata. J Exp Bot 2003; 54:1553 - 1564
- Boudart G, Jamet E, Rossignol M, Lafitte C, Borderies G, Jauneau A, et al. Cell wall proteins in apoplastic fluids of Arabidopsis thaliana rosettes: identification by mass spectrometry and bioinformatics. Proteomics 2005; 5:212 - 221
- Nembaware V, Seoighe C, Sayed M, Gehring C. A plant natriuretic peptide-like gene in the bacterial pathogen Xanthomonas axonopodis may induce hyperhydration in the plant host: a hypothesis of molecular mimicry. BMC Evol Biol 2004; 4:10
- Gottig N, Garavaglia BS, Daurelio LD, Valentine A, Gehring C, Orellano EG, et al. Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide-like protein to modify host homeostasis. Proc Natl Acad Sci USA 2008; 105:18631 - 18636
- Gottig N, Garavaglia BS, Daurelio LD, Valentine A, Gehring C, Orellano EG, et al. Modulating host homeostasis as a strategy in the plant-pathogen arms race. Commun Integr Biol 2009; 2:89 - 90
- Garavaglia BS, Thomas L, Gottig N, Dunger G, Garofalo CG, Daurelio LD, et al. A eukaryoticacquired gene by a biotrophic phytopathogen allows prolonged survival on the host by counteracting the shut-down of plant photosynthesis. PLoS ONE 2010; 5:8950
- Bolton MD. Primary metabolism and plant defensefuel for the fire. Mol Plant Microbe Interact 2009; 22:487 - 497
- Berger S, Sinha AK, Roitsch T. Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot 2007; 58:4019 - 4026
- Garavaglia BS, Thomas L, Zimaro T, Gottig N, Daurelio LD, Ndimba B, et al. A plant natriuretic peptide-like molecule of the pathogen Xanthomonas axonopodis pv. citri causes rapid changes in the proteome of its citrus host. BMC Plant Biol 2010; 10:51