Abstract
Autophagy is an intracellular bulk degradation/recycling system that turns over cellular constituents and also functions to degrade intracellular foreign microbial invaders by a process termed xenophagy (antimicrobial autophagy). We previously showed that intracellular group A Streptococcus (GAS) organisms are captured by xenophagosomes, then degraded following fusion with lysosomes. Very recently, we analyzed the molecular mechanism underlying xenophagosome/lysosome fusion and found that endocytic soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) are involved. Knockdown of the combinational SNARE proteins Vti1b and VAMP8 with siRNAs disturbed autophagic fusion with lysosomes, and cellular bactericidal efficiency was significantly diminished. Furthermore, knockdown of those SNAREs inhibited the fusion of canonical autophagosomes with lysosomes. In addition, important findings showed that Vti1b is derived from autophagic compartments, whereas VAMP8 originates from lysosomes. Together, these results strongly suggest that SNARE proteins Vti1b and VAMP8 mediate the fusion of antimicrobial and canonical autophagosomes with lysosomes, an essential event for autophagic degradation.
A large number of pathogenic bacteria are capable of invading non-phagocytic cells to colonize them intracellularly, after which they disseminate to other cells.Citation1 Previously, the endosomal degradation pathway was considered to the only target for prevention against such intracellular pathogens. Autophagy is an intracellular bulk degradation/recycling system for turnover of cellular constituents.Citation2 Notable recent findings in studies of autophagy have revealed this system to be a central component of the antimicrobial defense system, which captures and digests intracellular foreign microbial invaders by a process termed xenophagy (antimicrobial autophagy).Citation3 We previously showed that Group A Streptococcus (GAS) organisms, which produce a wide range of toxins, invade human epithelial cells via endocytosis, then escape from endosomes to the cytoplasm, after which they are entrapped within xenophagosomes and degraded upon fusion with lysosomes.Citation4 Canonical autophagosomes undergo a stepwise maturation process that consists of a fusion event with lysosomes, which allows autophagic vacuoles to acquire lysosomal proteases for degradation of the cargos.Citation5 In a similar manner, xenophagosomes that initially capture GAS include no lysosomal membrane protein 1 (LAMP1), though they subsequently become associated with LAMP1.Citation4 Finally, GAS organisms are degraded by xenophagosomes with lysosomal enzymes. Although xenophagosomes have been shown morphologically to be formed by the fusion of multiple small precursor structures,Citation6 the mechanism of fusion with lysosomes remains far from clear.
Xenophagosome-Lysosome Fusion
Soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) are known to be major players in the final stage of docking and subsequent fusion of diverse vesicle-mediated transport events.Citation7 Thus, we investigated whether they are involved in xenophagosome-lysosome fusion.Citation8 First, we examined if xenophagosomes are localized within various SNARE proteins, including endosome SNAREs (VAMP8 and Vti1b), endoplasmic reticulum-residing SNAREs, and trans-Golgi network-residing SNAREs, in GAS-infected human epithelial cells. GFP puncta of xenophagosomes were found to colocalize with those of VAMP8 and Vti1b, whereas knockdown of VAMP8 and Vti1b with siRNAs significantly inhibited the colocalization of xenophagosomes with lysosomes (). Furthermore xenophagosome formation was not affected by knockdown of VAMP8 and Vti1b, and the invasive efficiency of GAS into cells was not altered. In contrast, bactericidal efficiency was significantly diminished in SNARE-depleted cells. These results indicate that VAMP8 and Vti1b are directly involved in the mechanism of fusion between xenophagosomes and lysosomes.
Canonical Autophagosome-Lysosome Fusion
We also analyzed the effects of SNARE-knockdown on canonical autophagy. The microtubule-associated protein 1 light chain 3 (LC3) is an autophagosome-specific membrane marker in mammalian cellsCitation9 and expressed in 2 forms; LC3-I, which resides in the cytosol, and LC3-II, which is associated with autophagosomes.Citation2 LC3-II structures disappear for a few hours following conversion from LC3-I to LC3-II, due to degradation of LC3-II by autolysosomes as well as detachment from autolysosomes. However, GFP-LC3 puncta have been observed to be unchanged in VAMP8- and Vti1b-depleted cells after starvation to induce autophagy, indicating that autophagic degradation is inhibited due to fusion failure. A recent study showed that GFP loses its fluorescence in acidic conditions and is subsequently degraded by autolysosomes, whereas monomeric red-fluorescence protein (mRFP) remained stable under degradation conditions.Citation10 Therefore, we used mRFP-LC3 as well as mRFP-GFP in tandem with fluorescent-tagged LC3 (tfLC3) to analyze the distributions of LC3 proteins in canonical autophagosomes. mRFP puncta were found to be inconsistently merged with those of GFP in the control cells after starvation, indicating that GFP signals were attenuated in the autolysosomes, whereas mRFP remained. In contrast, those puncta were clearly colocalized in VAMP8- and Vti1b-depleted cells, indicating a negligible attenuation of GFP, and inhibition of fusion between autophagosomes and lysosomes in those cells. Knockdown of these SNAREs also depleted lysosomal enzymes in autophagosomes. Thus, VAMP8 and Vti1b are also involved in canonical autophagosome-lysosome fusion.
Origin of VAMP8 and Vti1b for Fusion
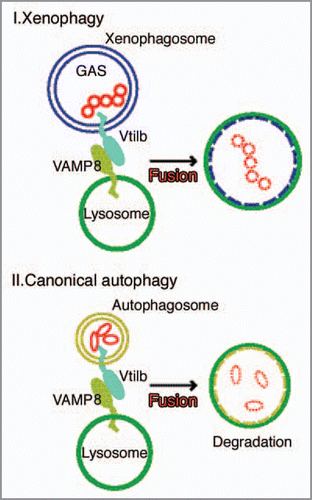
It is notable that a combination of endocytic SNAREs, which are involved in homotypic fusion within late endosomes, also functions in autophagy. Another interesting finding is that xenophagosomes possess Vti1b from an early stage, indicating that Vti1b is derived from xenophagosomes prior to fusion with lysosomes, whereas VAMP8 puncta are negligibly merged with xenophagosomes before fusion, then gradually become colocalized with them over time. VAMP8 originates from lysosomes and is recruited to autophagic compartments prior to lyosomal fusion ().
We found that xenophagosomes and autophagosomes share the same SNARE machinery to fuse with lysosomes, even though antimicrobial autophagy against GAS differs from canonical autophagy in several other aspects, such as size, morphology and conditions required for initiation.Citation3,Citation4 Our results are the first to show that VAMP8 and Vti1b in combination mediate autophagic fusion with lysosomes in mammals. Autophagy has various physiological roles and there is growing recognition that its impairment may be a common pathway for many types of mammalian disorders. In the future, various novel investigations will be needed to unravel autophagic functional events.
Figures and Tables
Figure 1 Knockdown of VAMP8 and Vti1b inhibits colocalization of xenophagosomes with lysosomes. HeLa cells expressing GFP-LC3 were transfected with control siRN A, and VAMP8 and Vti1b siRN As. Following infection with GAS, the cells were fixed and incubated with anti-LAMP1 antibodies and observed with a confocal microscope. Cellular and bacterial DN A were stained with DAPI. LAMP1 failed to colocalize with GFP-LC3 in the SNARE -depleted cells. Boxed regions in the upper panels show magnifications of the lower panels. Bars indicate 5 µm.
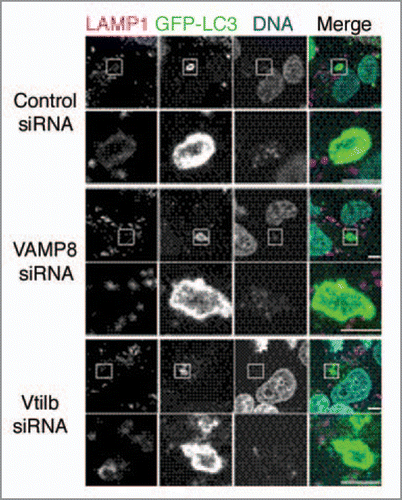
Figure 2 SNARE proteins VAMP8 and Vti1b fuse xenophagosomes/canonical autophagosomes with lysosomes. Intracellular GAS organisms are entrapped within xenophagosomes, then xenophagosomes undergo a stepwise maturation process that consists of a fusion event with lysosomes, which allows the autophagic vacuole to acquire lysosomal proteases. That fusion is mediated by a combinational complex of VAMP8 and Vti1b from xenophagosomes. Finally, GAS organisms are degraded by xenophagosomes (autolysosomes) possessing lysosomal degradation enzymes. The complex of VAMP8 and Vti1b also mediates the fusion between canonical autophagosomes and lysosomes. Vti1b originates from autophagic vacuoles, whereas VAMP8 is recruited from lysosomes to autophagic compartments prior to fusion.
Addendum to:
References
- Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 2004; 304:242 - 248
- Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun 2004; 313:453 - 458
- Yoshimori T, Amano A. Group A Streptococcus. A loser in the battle with autophagy. Curr Top Microbiol Immunol 2009; 335:217 - 226
- Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, et al. Autophagy defends cells against invading group A Streptococcus. Science 2004; 306:1037 - 1040
- Hamasaki M, Yoshimori T. Where do they come from? Insights into autophagosome formation. FEBS Lett 2010; 584:1296 - 1301
- Yamaguchi H, Nakagawa I, Yamamoto A, Amano A, Noda T, Yoshimori T, et al. An initial step of GAScontaining autophagosome-like vacuoles formation requires Rab7. PLoS Pathog 2009; 5:1000670
- Hong W. SNAREs and traffic. Biochim Biophys Acta 2005; 1744:493 - 517
- Furuta N, Fujita N, Noda T, Yoshimori T, Amano A. Combinational soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins VAMP8 and Vti1b mediate the fusion of antibacterial and canonical autophagosomes with lysosomes. Mol Biol Cell 2010; 21:1001 - 1010
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19:5720 - 5728
- Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007; 3:452 - 460