Abstract
Remodeling of synapses is a fundamental mechanism for information storage and processing in the brain. Previous studies showed that the endosomal pathway play a central role in synapse formation and plasticity. A popular model holds that recycling endosomes in dendrites provide the local intracellular pool of postsynaptic receptors for long-term potentiation (LTP), a widely studied cellular model for learning and memory formation. However, we are far from a complete understanding how endocytic receptor sorting and recycling is organized and coordinated in dendrites. Especially, the molecular mechanisms that couple specific endosomal trafficking routes during LTP are poorly understood. In a recent paper we discovered that the coiled-coil protein GRIP-associated protein-1 (GRASP-1) is a neuron-specific effector of the small GTPase Rab4 and key component of AMPA receptor recycling machinery in dendrites.1 GRASP-1 is essential for maintenance of spine morphology and important for LTP. GRASP-1 connects Rab4 and Rab11 recycling endosomal domains through the interaction with target (t)-SNARE syntaxin 13, which constitutes a new principle for regulating endosomal recycling. Here, we summarize our recently reported observations and further discuss their possible implications.
The molecular and cellular mechanisms that govern neuronal development and plasticity are highly complex, with many basic cellular pathways uniquely adapted to perform information processing. This is particularly evident in the neuronal trafficking machinery, where highly specialized mechanisms exist for localizing, maintaining and removing lipid and membrane proteins at the synapse (). The importance of these processes for synaptic function has been well documented for AMPA-type glutamate receptors (AMPAR) that represent the major excitatory neurotransmitter receptors. Redistribution of AMPAR in and out of the synapse has emerged as an important mechanism for synaptic plasticity and information storage in the brain.Citation2,Citation3 Increased delivery of AMPARs to the postsynaptic membrane leads to LTP, while net removal of AMPARs by internalization from the surface seems to underlie long-term depression (LTD).Citation4 Recent studies have suggested that dysregulation in AMPA receptor trafficking may underlie neurological diseases such as Alzheimer's disease and schizophrenia.Citation3,Citation5 Understanding the basic mechanisms that regulate AMPAR trafficking is thus of clinical significance.
A variety of highly conserved and ubiquitously expressed molecules including Rab GTPases Rab4, Rab5, Rab11,Citation6 molecular motors,Citation7–Citation9 and motor-adaptor/synaptic scaffolding proteinsCitation10,Citation11 are directly responsible for AMPA receptor trafficking and delivery. The Rabs act as binary switches that in their active form recruit/stabilize downstream effector networks and thereby function as organizers of discrete membrane microdomains. Rab5 controls transport to sorting endosomes,Citation12 whereas Rab4,Citation13 and Rab11,Citation14 regulate endosomal recycling back to the plasma membrane (). The communication and transport between sequentially organized Rab domains is thought to be mediated by proteins that are ‘shared’ by both domains. Bivalent effectors, such as Rabenosyn-5 can connect proximal Rab5 and distal Rab4 domains on early endosomes,Citation15 while the Mon1-Ccz1 complex through association with Rab5 and Rab7 can assist in the convergence of the Rab5 domain into a late endocytic Rab7 domain.Citation16 However, how recycling endosomal domains are coupled is not well understood. We recently isolated GRASP-1 as a neuron-specific Rab4 effector that is present on recycling endosomes and can connect Rab4 and Rab11 domains in a novel manner.Citation1 This link between the two recycling endosomal domains is important for normal synaptic function, since knock-down of GRASP-1 interferes with AMPAR recycling, synaptic plasticity and maintenance of spine morphology.
GRASP-1 was previously identified as a direct binding partner of glutamate receptor interacting protein 1 (GRIP1) and thought to be a guanine nucleotide exchange factor (GEF) for h-Ras.Citation17 Surprisingly, re-evaluation of the guanine nucleotide exchange activity showed no evidence for a role of GRASP-1 as GEF in vitro and in vivo.Citation1 Instead, our new data show that GRASP-1 together with Rab4 and the t-SNARE syntaxin 13 (also known as syntaxin 12) is associated with tubulovesicular recycling endosomes in neurons. GRASP-1 not only colocalizes with these proteins, but also directly binds to Rab4 and syntaxin 13 at the same time.Citation1 The N-terminus of GRASP-1 interacts with Rab4 while the C-terminus binds to syntaxin 13. Because syntaxin 13 is known is associate with the Rab11 domain, we propose that the GRASP1-syntaxin 13 complex forms a molecular ‘bridge’ between the Rab4 and Rab11 domains on recycling endosomes. Most likely one of the Rab11 effector molecules is engaged in interactions with either syntaxin 13 or GRASP-1 on the recycling endosomal membrane. The precise mechanistic aspects and dynamic properties of such an endosomal recycling “bridge” need to be worked out further.
Another aspect of the GRASP-1 complex concerns the state of syntaxin 13. Since endosomal SNARE proteins can be recycled through the plasma membrane for re-utilization,Citation18 it is likely that syntaxin 13 itself might be present as cargo in endosomal membranes with Rab4 on their cytoplasmic leaflet. Together they could act as a coincidence detector on recycling endosomal membranes,Citation1 since neither syntaxin 13 nor Rab4 alone is sufficient to localize GRASP-1 on endosomes. An alternative scenario features the idea that GRASP-1 targets the syntaxin 13 molecules that are present within the endosomal SNARE complex. In initial interaction assays with GRASP-1 and rat brain extracts, we co-isolated syntaxin 13 and specific vesicle (v)-SNAREs (unpublished observations). Given that the soluble NSF attachment protein (SNAP) was also present in the bound fraction, it seems likely that GRASP-1 interacts with the cis form of this SNARE complex on the same endosomal membrane domain. It is possible therefore that GRASP-1 might serve as an important contributor in the transition of a cis- to trans-SNARE complex that is required for membrane fusion during the maturation of recycling endosomes.
Since GRASP-1 is important for synaptic plasticity and dendritic spine remodeling, we anticipate that its function on recycling endosomes is controlled by neuronal activity. Indeed activation of NMDA receptors dramatically affects the somatodendritic distribution of GRASP-1.Citation17 Although GRASP-1 expression remains constant under these conditions, it is nearly quantitately translocated from endosomal membranes into the cytoplasm (unpublished observations). Importantly, the altered localization of GRASP-1 correlates with a shift in AMPA receptor localization from early and recycling endosomal organelles to degradative compartments of the endo-lysosomal system. A second, less well understood signaling pathway could irreversibly affect GRASP-1 distribution and function in neurons. GRASP-1 contains a C-terminal consensus sequence for caspase-3 cleavage.Citation17 In experimental models for apoptosis in vivo and in cultured neurons it was found that GRASP-1 was cleaved during development and ischemia.Citation19 Cleavage removes the physical connection between Rab4 and syntaxin 13 binding regions, as a consequence of which the N- and C-terminal GRASP-1 pieces relocate into the cytoplasm. It is not known whether the two fragments that are generated have functions unrelated to a role in regulating traffic through the recycling endosome system in neurons. However, this does seem less likely for the short C-termininal fragment, which is not stable and rapidly degraded over time.
The continuously improving biochemical and imaging techniques will greatly facilitate the discovery of new neuron specific factors controlling the structural and functional plasticity of synapses. It will be rewarding in future research to work out the molecular principles integrating the specific mechanisms involving such proteins as GRASP-1,Citation1 GRIP1,Citation10,Citation11 NEEP21,Citation20 and PICK-1,Citation21 in AMPAR trafficking, with the highly conserved and general components regulating the core endocytic pathways. We anticipate that this application of basic cell biological methods to approach neuronal function will generate many new ideas and insights in the fascinating processes underlying synaptic plasticity mechanisms.
Figures and Tables
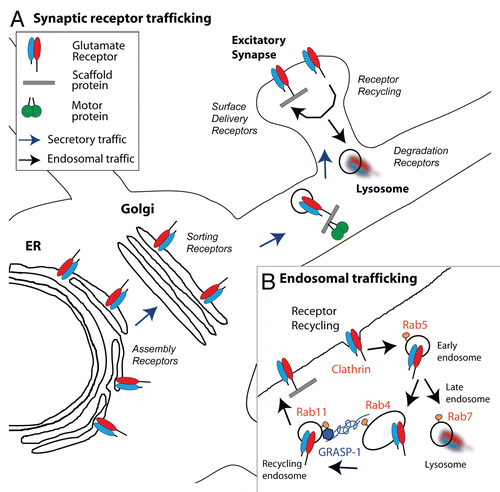
Figure 1 Synaptic receptor trafficking pathways in neuronal dendrites. (A) Schematic representation of the secretory and endosomal trafficking pathway of postsynaptic receptors. Glutamate receptor subunits are assembled in the endoplasmic reticulcum (ER) and are transported via the Golgi apparatus to the synaptic plasma membrane. The interaction between receptor and motor proteins often occurs through motor adaptor/synaptic scaffolding proteins. (B) Model for the endosomal membrane trafficking machinery in dendrites. The internalization of AMPA receptors into endocytic vesicles is mediated by clathrin acting on the lateral membrane within the spine. Rab5 controls transport to early endosomes (also called sorting endosomes) whereas Rab4 and Rab11 are involved in the regulation of endosomal recycling back to the plasma membrane. Re-entry of internalized AMPAR into the Rab4-Rab11 endosomal recycling route requires GRASP-1.
Acknowledgements
C.C.H. and P.v.d.S. are supported through grants from the Netherlands Organization for Scientific Research (NWO-ALW and NWO-CW). P.v.d.S. is supported by Netherlands Proteomics Center. CCH is supported by the Netherlands Organization for Health Research and Development (ZonMW-TOP), European Science Foundation (European Young Investigators (EURYI) Award), EMBO Young investigators programme (YIP), and Human Frontier Science Program Career Development Award (HFSP-CDA).
Addendum to:
References
- Hoogenraad CC, Popa I, Futai K, Martinez-Sanchez E, Wulf PS, van Vlijmen T, et al. Neuron specific rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol 2010; 8:1000283
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 2002; 25:103 - 126
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Biol Dev Biol 2007; 23:613 - 643
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron 2003; 40:361 - 379
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron 2009; 61:340 - 350
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature Revs Mol Cell Biol 2009; 10:513 - 525
- Correia SC, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, et al. Motor protein-dependent transport of AMPA receptors into spines during long term potentiation. Nature Neurosci 2008; 11:457 - 466
- Wang Z, Edwards JG, Riley N, Provance DWJ, Karcher R, Lim XD, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 2008; 135:535 - 548
- Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacIntosh FC, et al. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol 2010; 20:290 - 299
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, et al. Glutamate receptor interacting protein GRIP1 directly steers kinesin to dendrities. Nature 2002; 417:83 - 87
- Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M, Sheng M. GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nature Neurosci 2005; 8:906 - 915
- Bucci C, Parton R, Mather I, Stunnenberg H, Simons K, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 1992; 70:715 - 728
- van der Sluijs P, Hull M, Webster P, Goud B, Mellman I. The small GTP binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 1992; 70:729 - 740
- Ullrich O, Reinsch O, Urbe S, Zerial M, Parton R. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 1996; 135:913 - 924
- de Renzis S, Sönnichsen B, Zerial M. Divalent rab effectors regulate the sub-compartmental organization and sorting function of early endosomes. Nature Cell Biol 2002; 4:124 - 133
- Kinchen JM, Ravichandran KS. Identification of two evolutionary conserved genes regulating processing of engulfed apoptotic cells. Nature 2010; 464:778 - 782
- Ye B, Liao D, Zhang X, Zhang P, Dong H, Huganir R. GRASP-1: a neuronal rasGEF associated with the AMPA receptor/GRIP complex. Neuron 2000; 26:603 - 617
- Grote E, Hao JC, Bennett MK, Kelly RB. A targeting signal in vamp regulating transport to synaptic vesicles. Cell 1995; 81:581 - 590
- Ye B, Sugo N, Hurn PD, Huganir RL. Physiological and pathological caspase cleavage of the neuronal rasGEF GRASP-1 as detected using a cleavage site specific antibody. Neuroscience 2002; 114:217 - 227
- Steiner P, Sarria JCF, Glauser L, Magnin S, Catsicas S, Hirling H. Modulation of receptor recycling by neuron-enriched endosomal protein of 21 kDa. J Cell Biol 2002; 157:1197 - 1209
- Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron 2005; 47:407 - 421