Abstract
Regulation by signaling molecules of pathways involved in determining cell size and shape is fundamental to understand morphogenesis. In eukaryotic cells, Rho GTPases modulate cellular events by acting as molecular switches. GTPase Activating Proteins (GAPs) control the fine-tuning of Rho GTPase activity as downregulators that promote their inactive state. We use Schizosaccharomyces pombe as a model to unveil key mechanisms underlying processes of general significance. Rga4, one of the nine RhoGAPs present in the fission yeast, is a key factor in the control of cell polarity and morphogenesis by negatively regulating the activity of the essential Rho GTPase Cdc42. We have demonstrated that Rga4 is also a GAP for Rho2 GTPase, which acts upstream of the Pmk1 cell integrity MAP kinase pathway and positively regulates cell integrity and cell separation. Our findings suggest that Rga4 control of both Cdc42 and Rho2 function is rather independent, thus providing a good example of regulatory specificity. Additionally, we describe multiple GAPs that can downregulate Pmk1 activity in a Rho2-dependent and independent fashion. These studies corroborate the existence of a sophisticated regulatory network by which different RhoGAPs modulate differentially the activity of Rho GTPases, and the existence of different inputs for the Pmk1 cell integrity MAP kinase pathway.
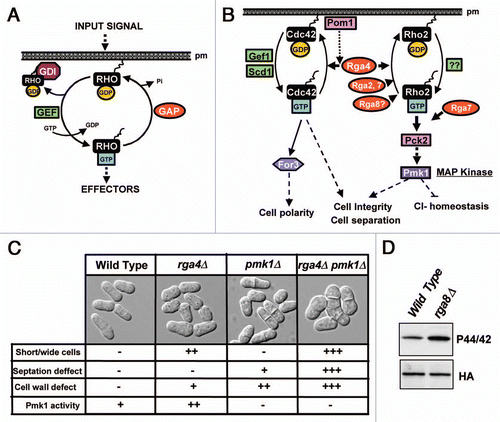
The Rho (Ras homologous) GTPases form a subgroup within the superfamily of small G proteins which is highly conserved among eukaryotes. These proteins play important cellular roles and are involved, among other key functions, in the regulation of actin and microtubule cytoskeleton, cell polarity, vesicle trafficking, cell cycle and gene expression.Citation1 They bind guanine nucleotides (GDP or GTP) and harbor intrinsic GTPase activity to hydrolyze the bound GTP to GDP and inorganic phosphate.Citation2 Considering the high number of biological processes regulated by Rho GTPases, it appears evident that their activity must be precisely regulated in both time and space. Remarkably, although Rho proteins show high affinity for binding either GTP or GDP, their intrinsic GTPase activity is low, and the exchange of GDP for GTP is rather inefficient.Citation3 The regulation of the activity of Rho GTPases includes three classes of proteins: Guanine Nucleotide Exchange Factors (GEFs), GDP Dissociation Inhibitors (GDIs), and GTPase Activating Proteins (GAPs)Citation4 (). GEFs interact with GDP-bound GTPases to promote their activation through dissociation and substitution of GDP by GTP (). GDIs bind Rho GTPases and inhibit both their binding to the membrane and their interaction with GEFs, thus sequestering them in the cytosol and favoring their maintenance in an inactive state (). GAPs activate intrinsic Rho GTPase activity by enhancing hydrolysis of GTP to GDT and inducing the GTPase to remain inactive (). The specificity of GAPs toward Rho is defined by the presence of a Rho GAP domain which comprises a conserved ∼150 amino acid sequence able to interact with Rho to downregulate its GTPase activity.Citation5
In all eukaryotic organisms the number of Rho GAPs exceeds that of Rho GTPases. For example, the human genome encodes approximately 70 GAPs which negatively regulate about 20 Rho GTPases.Citation4 A consequence of this overabundance of Rho GAPs is that they are “promiscuous”, so that a given GAP downregulates more than one GTPase. However, the contrary also applies, and a single Rho GTPase can be regulated by several GAPs. This situation highlights the complexity and multiplicity of the mechanisms involved in the GAP-dependent negative regulation of Rho activity.
Our team works with the model organism Schizosaccharomyces pombe, which is commonly known as fission yeast. This simple eukaryote appears phylogenetically closer to higher eukaryotes than to other yeasts.Citation6 In fact, a great body of experimental evidence confirms the existence of significant functional homology among the intracellular signaling pathways of S. pombe and those present in higher cells. Such homology, together with its flexibility to genetic manipulation and the availability of its complete genome sequence, converts the rod-shaped yeast into a model system of general significance and quite suitable to investigate different cellular processes such as morphogenesis, cell cycle progression and stress-mediated responses.Citation7,Citation8
The fission yeast proteome contains six Rho GTPases, namely, Rho1 to Rho5 and Cdc42.Citation9 Cdc42 is an essential GTPase which modulates polarized growth and morphogenesis in S. pombe cells.Citation10 In this organism Cdc42 plays a critical role in the assembly of actin by modulating both the activation and localization of For3, a formin responsible for actin cable formationCitation11,Citation12 (). Another GTPase, Rho2, is not essential but it participates in the biosynthesis of the cell wall (1–3)β-D-glucan and also activates Pck2, a protein kinase C ortholog that is required for proper (1–3)α-D-glucan biosynthesis.Citation13,Citation14 Importantly, Rho2 and Pck2 are essential for both the basal activity and the activation of the cell integrity Mitogen Activated Protein Kinase (MAPK) pathwayCitation15,Citation16 (). During the last years we have been investigating this signaling cascade, whose key element is MAP kinase Pmk1, an ERK-type kinase that becomes activated in response to a variety of external stimuli, and that is involved in the maintenance of the cell integrity as well as in the regulation of cell separation (cytokinesis) and chloride homeostasis ().Citation17–Citation19
In fission yeast the existing GAPs also outnumber the Rho GTPases. We initially found that Rga2, one of the nine Rho GAPs present in S. pombe (Rga1 to Rga9),Citation20 is a specific Rho2 GAP which negatively regulates the activity of the Pmk1 MAPK pathway.Citation21 However, our data strongly suggested that Rga2 was not the only Rho2 GAP able to downregulate this signaling cascade. Indeed, after performing a biochemical screening we found that Pmk1 basal activity increased in mutants lacking either Rga4 or Rga7 GAPsCitation22 (). The possibility that Rga4 might act as a Rho2 GAP was of particular interest because a recent report had described evidence supporting its role as a Cdc42 GAP involved in the control of cell diameter and symmetry in the fission yeast ().Citation23 Cells from a mutant strain lacking Rga4 are shorter and wider than wild type cells, which grow by elongation at the cell poles in an asymmetric manner (). On the contrary, Rga4 overproduction induces narrowing at the cell ends.Citation23,Citation24 Considering that Cdc42 localizes mainly at the growing poles, whereas Rga4 is excluded from the growing areas and localizes to the cell sides, it has been hypothesized that Rga4 would play a critical role in the polarized distribution of active (GTP-loaded) Cdc42 by defining the cortical region where the GTPase is kept in an inactive state.Citation23
By performing two-hybrid assays and co-immunoprecipitation studies we first found that Rga4 interacts in vivo with Rho2 GTPase, although this association is not as strong as that observed with Cdc42.Citation22 However, in vitro and in vivo analysis showed that the amount of GTP-bound Rho2 increased significantly in a strain lacking Rga4 while the binding was reduced in Rga4-overexpressing cells. This result confirmed that Rga4 is a Rho GAP which specifically downregulates Rho2 in the fission yeast ().Citation22 Notably, the increased Pmk1 phosphorylation observed in cells lacking Rga4 was dependent on the presence of Rho2, indicating that this GTPase is the target for Rga4 as negative regulator of the cell integrity MAPK pathway ().Citation22 Additionally, the demonstration of the bifunctional nature of Rga4 as an in vivo GAP for Cdc42 and Rho2 proved to be an excellent opportunity to determine whether this GAP acts independently on two different GTPases or participates in some type of crosstalk. This issue was clarified by performing a comparative analysis of several phenotypic features in wild type cells, and in cells from strains lacking either Rga4, Pmk1, or both proteins. Remarkably, the increased cell width of cells lacking Rga4 not only was not abrogated by simultaneous deletion of Pmk1 but was aggravated (), supporting the conclusion that the Rga4-Cdc42 regulation of cell diameter is independent of the control of Pmk1 activity brought about by Rga4 through Rho2 ().Citation22 Fission yeast cells devoid of Pmk1 activity showed cell wall defects and a defective cell separation characterized by the presence of many septating cells, some of them containing multiple septa (). Interestingly, simultaneous deletion of Rga4 and Pmk1 aggravated both the cell wall and cell separation defects (), suggesting that Rga4 positively regulates the cell wall integrity and the cell separation through Cdc42, independent of the Pmk1 pathway.
One important question that arises from these studies is how the Rga4 regulatory specificity is achieved. Several mechanisms like phosphorylation, lipid binding, subcellular localization, proteolytic degradation and protein-protein interactions have been shown to modulate the activity of different RhoGAPs in eukaryotes.Citation4 In this context, Rga4 is a phosphoprotein, and its phosphorylation state is affected by Pom1, a protein kinase from the DYRK-family.Citation23 Moreover, Pom1 is essential for proper localization of Rga4 to ensure bipolar localization of GTP-bound, active Cdc42.Citation23 However, we have found that Pom1 modulates Pmk1 activity in a Rga4-independent fashion.Citation22 Hence, an attractive possibility to explain the specificity of action within the apparent promiscuity of Rga4 would be that in fission yeast two subsets of Rga4 independently regulate the activity of Cdc42 (Pom1-dependent) and Rho2 (Pom1-independent) GTPases. Additionally, differential subcelular localization of Rga4 (plasma membrane, endosomes,…) might be important to specify its target GTPase.
Our results indicate that the regulation of Pmk1 MAPK pathway by Rho GAPs is even more complicated since besides Rga2 and Rga4, another GAP, Rga7, negatively regulates this pathway. Interestingly, Rho2 does not seem to be the only target for Rga7, because the absence of Rho2 did not fully alleviated Pmk1 hyperactivation in Rga7 deleted cells.Citation22 Additionally, we have recently discovered that the lack of Rga8, which had been previously described to be a GAP for Rho1,Citation25 induces a clear increase in Pmk1 phosphorylation (). Hence, it is possible that Rga8 could be a GAP for both Rho1 and Rho2. Given the structural and functional complexity of Rho-GAPs in higher eukaryotes, further studies employing fission yeast will provide a good model to elucidate the mechanisms which govern both the specific regulation of several Rho GTPases by a single GAP and how several GAPs differentially modulate the activity of a single GTPase.
Figures and Tables
Figure 1 (A) Three classes of proteins, Guanine Nucleotide Exchange Factors (GEFs), GDP Dissociation Inhibitors (GDIs), and GTPase Activating Proteins (GAPs) are responsible for the cyclic regulation of Rho GTPase activity. (B) A model showing the known GEFs and GAPs involved in the regulation of Cdc42 and Rho2 GTPases in fission yeast. No specific GEFs for Rho2 have been identified yet. Rga4 is the only known GAP for Cdc42, whereas Rga2, Rga4, Rga7 and probably, Rga8 are Rho2 GAPs. Pom1 kinase might provide a role in determining Rga4 specific signalling through Cdc42. The formin For3 is a direct target of Cdc42 in the regulation of cell polarity, whereas Rho2 functions upstream protein kinase C to activate Pmk1 MAPK, which is the core element of the cell integrity pathway regulating ion homeostasis, cell integrity and separation. (C) Distinct features were scored (“+++”, very strong; “++”, strong; “+”, weak; “−”, not detected) in wild type cells and in fission yeast mutants lacking Rga4 (rga4Δ), Pmk1 (pmk1Δ) and both Rga4 and Pmk1 (rga4Δ pmk1Δ) after observation by differential interference contrast (DIC) microscopy and photographed. Cell width, cell wall defects, and a defective cell separation were exacerbated by simultaneous deletion of Rga4 and Pmk1 as compared to single mutants, supporting the regulatory model proposed in (B). (D) Rga8 GAP is a negative regulator of Pmk1 phosphorylation. Cells from wild type and a mutant strain lacking Rga8 (rga8Δ) and carrying a HA6H-tagged chromosomal version of the pmk1+ gene were grown to mid-log phase and Pmk1-HA6H was purified by affinity chromatography. Activated Pmk1 was detected by immunoblotting with anti-(phosho-p44/42) antibody and total Pmk1 was detected with anti-HA antibody as loading control.
Acknowledgements
This work was supported by grants BFU2007-60675 and BFU2008-01653 from MICINN, 08725/PI/08 from Fundación Séneca (Región de Murcia), Spain and GR231 from Castilla y Leon, Spain.
Addendum to:
References
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 2007; 29:356 - 370
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature 1991; 349:117 - 127
- Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci 2005; 118:843 - 846
- Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell 2007; 99:67 - 86
- Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol 2003; 13:13 - 22
- Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stewart A, et al. The genome sequence of Schizosaccharomyces pombe. Nature 2002; 415:871 - 880
- Gacto M, Soto T, Vicente-Soler J, Villa TG, Cansado J. Learning from yeasts: intracellular sensing of stress conditions. Int Microbiol 2003; 6:211 - 219
- Gould KL. Protocols for experimentation with Schizosaccharomyces pombe. Methods 2004; 33:187 - 188
- Arellano M, Durán A, Pérez P. Rho1 GTPase activates the (1–3)beta-D-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J 1996; 15:4584 - 4591
- Miller PJ, Johnson DI. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol Cell Biol 1994; 14:1075 - 1083
- Martin SG, Rincón SA, Basu R, Pérez P, Chang F. Regulation of the forming for3p by cdc42p and bud6p. Mol Biol Cell 2007; 18:4155 - 4167
- Rincón SA, Ye Y, Villar-Tajadura MA, Santos B, Martin SG, Pérez P. Pob1 participates in the Cdc42 regulation of fission yeast actin cytoskeleton. Mol Biol Cell 2009; 20:4391 - 4399
- Calonge TM, Nakano K, Arellano M, Arai R, Katayama S, Toda T, et al. Schizosaccharomyces pombe rho2p GTPase regulates cell wall alpha-glucan biosynthesis through the protein kinase pck2p. Mol Biol Cell 2000; 11:4393 - 4401
- Katayama S, Hirata D, Arellano M, Pérez P, Toda T. Fission yeast alpha-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J Cell Biol 1999; 144:1173 - 1186
- Ma Y, Kuno T, Kita A, Asayama Y, Sugiura R. Rho2 is a target of the farnesyltransferase Cpp1 and acts upstream of Pmk1 mitogen-activated protein kinase signaling in fission yeast. Mol Biol Cell 2006; 17:5028 - 5037
- Barba G, Soto T, Madrid M, Núñez A, Vicente J, Gacto M, et al. Activation of the cell integrity pathway is channelled through diverse signalling elements in fission yeast. Cell Signal 2008; 20:748 - 757
- Toda T, Dhut S, Superti-Furga G, Gotoh Y, Nishida E, Sugiura R, et al. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol Cell Biol 1996; 6:6752 - 6764
- Zaitsevskaya-Carter T, Cooper JA. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S. pombe. EMBO J 1997; 16:1318 - 1331
- Madrid M, Soto T, Khong HK, Franco A, Vicente J, Pérez P, et al. Stress-induced response, localization and regulation of the Pmk1 cell integrity pathway in Schizosaccharomyces pombe. J Biol Chem 2006; 281:2033 - 2043
- Nakano K, Mutoh T, Mabuchi I. Characterization of GTPase-activating proteins for the function of the Rho-family small GTPases in the fission yeast Schizosaccharomyces pombe. Genes Cells 2001; 6:1031 - 1042
- Villar-Tajadura MA, Coll PM, Madrid M, Cansado J, Santos B, Pérez P. Rga2 is a Rho2 GAP that regulates morphogenesis and cell integrity in S. pombe. Mol Microbiol 2008; 70:867 - 881
- Soto T, Villar-Tajadura MA, Madrid M, Vicente J, Gacto M, Pérez P, et al. Rga4 modulates the activity of the fission yeast cell integrity MAPK pathway by acting as a Rho2 GAP. J Biol Chem 2010; 285:11516 - 11525
- Tatebe H, Nakano K, Maximo R, Shiozaki K. Pom1 DYRK regulates localization of the Rga4 GAP to ensure bipolar activation of Cdc42 in fission yeast. Curr Biol 2008; 18:322 - 330
- Das M, Wiley DJ, Medina S, Vincent HA, Larrea M, Oriolo A, et al. Regulation of cell diameter, For3p localization, and cell symmetry by fission yeast Rho-GAP Rga4p. Mol Biol Cell 2007; 18:2090 - 2101
- Yang P, Qyang Y, Bartholomeusz G, Zhou X, Marcus S. The novel Rho GTPase-activating protein family protein, Rga8, provides a potential link between Cdc42/p21-activated kinase and Rho signaling pathways in the fission yeast, Schizosaccharomyces pombe. J Biol Chem 2003; 278:48821 - 48830