Abstract
Exosomes are specialized membranous nano-sized vesicles derived from endocytic compartments that are released by many cell types. Microvesicles are distinctive from exosomes in that they are produced by shedding of the plasmamembrane and usually larger in size (>1µm). Exosome biogenesis involves the tightly controlled process of inward budding from the limiting membrane of multivesicular bodies (MVBs). This results in numerous intraluminal vesicles in the lumen of MVBs that contain distinct protein repertoires. It has been suggested that microvesicles shed by certain tumor cells hold functional messenger RNA (mRNA) that may promote tumor progression. We discovered that purified exosomes contain functional microRNAs (miRNAs) and small RNA, but detected little mRNA. Although a clear and decisive distinction between microvesicles and exosomes cannot be made and different subsets of exosomes exist, we speculate that exosomes are specialized in carrying small RNA including the class 22-25 nt regulatory microRNAs. To demonstrate this we developed a co-culture system and found that exosomes are continuously secreted and transferred from Epstein Barr virus (EBV)-infected cells to uninfected neighboring cells. Throughout exosome transfer, the exogenous EBV-encoded miRNAs were delivered to subcellular sites of miRNA-mediated gene repression. Additionally, we found evidence that mature miRNAs are transferred between circulating cells in humans, since we detected EBV-miRNAs in non-infected cells in the peripheral blood of patients that include monocytes and T cells. In this addendum we discuss these findings in the context of recently published papers that advanced our current knowledge of exosome physiology, (mi)RNA function and intercellular RNA transfer. Based on this information we propose that an intercellular (miRNA-based) mode of signal transmission may be well suited in controlling space-confined processes such as the initiation of immune responses in the secondary (peripheral) lymphoid tissues or in a tumor microenvironment. Deciphering the molecular mechanism(s) that control small RNA loading into exosomes and transfer to recipient cells in vitro will provide new evidence for the physiological relevance of vesicle-mediated intercellular communication in vivo.
Communication between individual cells and tissues as a whole was long conceived to be primarily mediated through the secretion of soluble factors including hormones, cytokines and chemokines that bind to specialized receptors expressed on the surface of target cells. It has now become apparent that additional, arguably more sophisticated methods of cell-cell communication exist, that in potential vastly exceed the multitude of signals that can be delivered through soluble factors alone. Besides the well studied signalling pathways that are activated upon ‘classic’ cell-cell contact formation, numerous additional mechanism of intercellular communication have now been proposed.Citation1 These can roughly be separated into two categories; those that require some form of cell-cell contact and those that do not (contact-independent). The transfer of functional proteins appears to be surprisingly common, in particular between cells of the immune system.Citation2,Citation3 Viruses with a clear tropism for immune cells such as human immunodeficiency virus (HIV) and Epstein Barr Virus (EBV) have evolved strategies to exploit these pathways to ensure viral persistence that inadvertently may cause virus-associated disease.Citation4–Citation7
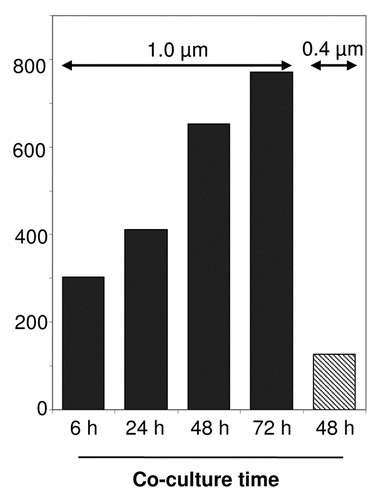
While the vesicle-mediated transfer of functional proteins between cells has been established, the notion of horizontal transfer of functional RNA molecules via this method has yet to reach this point. Several independent studies employing various cell-systems confirmed the idea that cell-derived vesicles carry and deliver functional messenger (m)RNA to recipient cells in vitro.Citation8–Citation11 In apparent contrast to these previous studies, we found in EBV-infected lymphoblasts (LCLs), that a defined class of vesicles of late-endosomal origin dubbed exosomes, selectively contain small RNAs and little if any mRNA.Citation12 This was not due to differences in susceptibility to degradation because RNAse treatment of purified exosomes completely removed any contaminating ribosomal RNA, yet small RNA was unaffected by the RNAse treatment. In addition, we showed that exosomes deliver functional microRNAs (miRNAs) in physiologically relevant copynumbers to recipient cells, leading to a miRNA-mediated repression of target genes. Our findings agree with studies describing functional transfer of EBV-miRNAs between EBV-infected B cells and T cells as recipients.Citation13 Interestingly, in these studies cell-cell contact was required for transfer. Using a co-culture device with a 0.4 µm semi-permeable membrane physically separating the B cells and T cells (presumably allowing secreted exosomes to diffuse freely), transfer of small RNAs to the recipient T cells was inefficient compared to conditions where B- and T cells were allowed to form conjugates. Yet, efficient transfer was only observed for small RNAs, while mRNA transfer was highly inefficient.Citation13 Because the possibility of vesicles contributing small RNA transfer was not completely ruled out, it is possible that exchange between contacting B and T cells of small RNAs is actually quite efficient via exosomes. Indeed, in our transwell co-culture system we observed highly efficient exosome-mediated EBV-miRNA transfer between EBV positive producer B cells and recipient monocyte-derived dendritic cells as recipients. For efficient transfer to occur a transwell device with a membrane pore-size of 1.0 µm was required (). Transfer was markedly impaired using a 0.4 µm semipermeable membrane, confirming the findings by Rechavi and colleagues.Citation13 Perhaps the assumption that secreted exosomes disseminate freely through a 0.4 um semi-permeable membrane is incorrect. Although single exosome-size ranges from 30–100 nm, it is currently not known whether exosomes function as single entities or if exosomes function as small clusters or aggregates (). Nevertheless, these independent studies clearly indicate that EBV-miRNAs are able to function in multiple, non-infected, recipient cell types that have a role in the life-cycle of EBV, underscoring the notion that infection with a viral agent such as EBV may have far greater influence on it's host and host cell-cell communication than originally perceived.
It is evident that crucial details in our understanding of exosome secretion and internalization are still lacking. However, an important step forward are two recent discoveries indicating that exosome secretion is dependent on specific Rab GTPases that function as molecular switches. This large family of small GTPases and their mediators, the guanine exchange factors (GEFs) and GTPase-activating proteins (GAPs) have an important role in intracellular vesicle transport. Notably the RAB27 isoforms A and B as well as RAB35 have now clearly been implicated in the regulation of exosome physiology.Citation14,Citation15 Indeed, our unpublished proteomic data indicate that RAB35 is associated with and enriched in purified exosomes from various cell backgrounds. These findings are consistent with a growing appreciation for the generic role of the RAB GTPase system in vesicular membrane transport inside the cell.Citation16 Despite a relatively detailed understanding of intracellular vesicle transport and the new advances into exosome secretion, a mechanism that specifically directs any RNA molecule into multivesicular bodies (MVBs) and exosomes has yet to be proposed. The molecular machinery that generates intraluminal vesicles (ILVs) in maturing endosomes is regulated, in part, by the evolutionary endosomal sorting complex required for transport (ESCRT). Members of the ESCRT complex are also responsible for selective protein sorting into MVBs. Other mechanisms that generate ILVs may depend on the constitution of the limiting membrane of MVBs.Citation17 The first concrete evidence that the loading of miRNAs into exosomes may not be a random event, but could be controlled by specific proteins involved in the (mi) RNA network, was recently provided by Gibblings and colleagues.Citation18 These authors showed the presence of AGO2 protein and a striking enrichment of GW182 in purified monocyte-derived exosome-like vesicles, suggestive of the selective sorting of GW182 into exosomes. Possibly, the association with GW182 directs EBV and cellular miRNAs into ILVs and exosomes of the EBV-infected LCLs. Although Gibblings et al.Citation18 found specific mRNAs in exosomes, miRNA-repressible mRNAs (presumably not housekeeping genes) were under-represented in exosomes consistent with the idea of selective enrichment of RNA in exosomes. Overall, our studies add to the possibility that virus-produced miRNAs co-opt an existing molecular pathway for specific and selective loading of miRNAs into MVBs and secretion through exosomes, most notably since cellular miRNAs have now been implicated in the exosomal transfer as well.Citation12,Citation19
In contrast to these improvements in our understanding of exosome physiology and RNA transfer, the biogenesis of plasma membrane-derived microvesicles and their RNA content is less well understood. In a first attempt to understand this process better, the group of D'schouza-Schorey convincingly showed that activation of ARF6 is critical for an actomyosin-based membrane ‘abscission mechanism’ that regulates the shedding of selected cellular components from the plasma membrane.Citation20 Although these vesicles were not analyzed for their RNA content, others have shown that microvesicles shed by tumor cells in vitro and in vivo, are considerably larger in size compared to exosomes and generally contain mRNA molecules when analyzed by bioanalyzer.Citation8 Various stabilized mRNA molecules are transported into and asymmetrically localized within the cytoplasm of polarized cells, hence a molecular machinery may exist that directs specific RNA molecules to sites of vesicle shedding at the plasma membrane.
In conclusion, vesicle-mediated RNA transfer has unequivocal advantages over ‘classical’ intercellular communication mediated by soluble factors. Instead of one single messenger, exosomes could deliver multiple messages at once and in case of functional miRNA transfer, functionally related genes could be suppressed simultaneously, allowing immediate control over neighboring cells. Perhaps no wonder that EBV, an extremely successful pathogen infecting 90% of the world population and persists for life within memory B-cells, appears to have co-opted this communication pathway for it's own benefit.
Figures and Tables
Figure 1 Exosome-mediated small RNA transfer in a co-culture system. A co-culture transwell device with 5,00,000 EBV positive exosome producer cells (LCL) and 1,00,000 dendritic cells (immature monocyte-derived dendritic cells) as recipients, were co-cultured for the indicated times and separated by a semi-permeable membrane with either a 1.0 µm (black bars) or 0.4 µm pore-size (grey bar). The bars represent the relative amount of EBV-encoded small RNA (EBERs), detected by semi-quantitative RT-PCR in the recipient cells as an indication for small RNA transfer.
Addendum to:
References
- Belting M, Wittrup A. Nanotubes, exosomes and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J Cell Biol 2008; 183:1187 - 1191
- Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol 2007; 73:238 - 243
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; In press
- Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 2009; 113:1957 - 1966
- Middeldorp JM, Pegtel DM. Multiple roles of LMP1 in Epstein-Barr virus induced immune escape. Semin Cancer Biol 2008; 18:388 - 396
- Xu W, Santini PA, Sullivan JS, He B, Shan M, Ball SC, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol 2009; 10:1008 - 1017
- Nguyen DG, Booth A, Gould SJ, Hildreth JE. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem 2003; 278:52347 - 52354
- Skog J, Wurdinger T, van RS, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10:1470 - 1476
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654 - 659
- Weber C, Schober A, Zernecke A. MicroRNAs in arterial remodelling, inflammation and atherosclerosis. Curr Drug Targets 2010; In press
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 2005; 113:752 - 760
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA 2010; 107:6328 - 6333
- Rechavi O, Erlich Y, Amram H, Flomenblit L, Karginov FV, Goldstein I, et al. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev 2009; 23:1971 - 1979
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12:19 - 30
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 2010; 189:223 - 232
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513 - 525
- Simons M, Raposo G. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009; 21:575 - 581
- Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol 2009; 11:1143 - 1149
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010; In press
- Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 2009; 19:1875 - 1885