Abstract
The lateral organizations of receptors in the cell membrane display a tremendous amount of complexity. In some cases, receptor functions can be attributed to specific spatial arrangements in the plasma membrane. We recently found that one member of the largest subfamily of receptor tyrosine kinases (RTKs), EphA2, is organized over micrometer length scales by the cell’s own cytoskeleton, and that this can regulate receptor signaling functions. Spatial organization of the receptor was found to be highly associated with invasive character, and mechanical disruption of receptor organization altered key down-stream events in the EphA2 signaling pathway. In this Addendum article, we put forth possible models for why EphA2 and other receptors may employ mechanical and spatial inputs mediated by the cytoskeleton. We speculate that this class of input may be common, and contributes to the intricacies of cellular signaling.
Receptor Spatial Organization
The spatial organization of receptors in the cell membrane spans multiple length scales, from the molecular to the size of the cell itself. Signaling assemblies consisting of tens, to tens of thousands of molecules can apparently function as cooperative units. Hierarchical organization of signaling receptors can directly feed into signaling pathways to regulate collective cell signaling outcomes.Citation1–Citation3 For example, T-cell receptor activation was found to be dependent on the spatial organization within the immunological synapse,Citation1,Citation4–Citation9 and in a recent report we recently found that the EphA2 receptor tyrosine kinase (RTK) pathway can be modulated based on receptor translocation.Citation3
There are distinct biophysical mechanisms that regulate receptor spatial organization and associated biochemical functions. The most commonly studied is direct protein-protein interaction. For example, the ligand-induced dimerization of RTKs is widely considered as the prototypical mechanism for their activation.Citation2,Citation10–Citation13 Another effecter that influences protein organization is lipid-membrane driven separation of proteins into discreet assemblies. The formation of such lipid membrane compartments may be based on the immiscibility of specific lipid components in the plasma membraneCitation14–Citation16 or mechanical bending effects at an intermembrane junction.Citation17 A third cellular regulator of protein organization is the network of cytoskeleton filaments which can act as scaffolds with the aid of adaptor proteins for corralling or directly moving receptors across the cell membrane.Citation1,Citation3,Citation7,Citation18 The interplay between these mechanisms exerts hierarchal and dynamic control of receptor organization and cell function. The role of the cytoskeleton is typically studied in the context of adhesion proteins such as integrins, and its role in the arrangement of free floating membrane proteins is poorly defined.Citation18,Citation19 This is because the connectivity between free floating receptors and the cytoskeleton is not clear and little is known about these associations.
The EphA2 Signaling Pathway
RTKs play important roles in receiving and amplifying signals from other cells and from the immediate environment. The Eph family of receptors constitute the largest subfamily of RTKs, and these contribute to cellular development and morphogenesis in a wide range of tissues. Abnormal expression and function of the EphA2 receptor is implicated in a range of human malignancies including breast, lung and ovarian cancers. In particular, 40% of human breast cancers overexpress EphA2, which is associated with a poor prognosis and the development of drug resistance.Citation20–Citation22
The ligand to EphA2 is a membrane-associated GPI-linked protein expressed on the surface of adjacent cells.Citation22,Citation23 Because both the ligand and receptor are in membranes, EphA2 binding and activation can only proceed through direct physical contact between cells. Structural studies of EphA receptors indicate that ligand-binding can lead to dimerization and the formation of higher order aggregates.Citation22,Citation23 Clustering of Eph-ephrin complexes is thought to be enhanced by specific domains.Citation24 These include the fibronectin type III repeats, the SAM domain of the Eph receptors and by PDZ domain proteins.Citation25 Ligand-induced clustering of the EphA2 receptor results in autophosphorylation and recruitment of downstream signaling molecules through Shc and Grb2 adaptor proteins. Receptor activation leads to stimulating the PI3K, Akt and MAPK pathways and will result in recruitment of the c-Cbl adaptor protein and a disintegrin and metalloprotease 10 (ADAM10) which regulate signaling through receptor degradation.
As is the case with most studies on such juxtacrine signaling systems, activation of EphA2 is often achieved with soluble ligands that are pre-clustered. We hypothesized that ephrin-A1 bound to synthetic lipid membranes would provide for a better mimic of the natural cell-cell junction geometry and might reveal additional features of this signaling process.Citation3 This interface presents active ephrin-A1 ligand molecules that are fluid in two dimensions and thus captures some of the native geometry. We found that membrane-bound ephrin-A1 triggers the EphA2 receptor on living cells and allows for quantifying receptor translocation. Such quantitative measurements are difficult in live cell couples, and therefore have not been explored in detail.
Seeking Signals: Cytoskeleton Transport of Ligand-Bound EphA2
Using live-cell fluorescence microscopy techniques we found that the EphA2 receptor rapidly formed clusters as a result of ligand binding. Clusters grew and coalesced until they were transported to the center of the cell-supported membrane junction. The motion of the EphA2-ephrin-A1 clusters was highly correlated to the motions of the actin cytoskeleton. This was measured using two-color total internal reflection fluorescence microscopy (TIRFM) tracking of ephrin-A1 and enhanced green fluorescent (EGFP)-actin. Eph receptors are known to play a role in remodeling the actin cytoskeleton and to elicit actomyosin contraction through the Rho family of guanosine triphosphate hydrolases (GTPases).Citation24 Ephrin-A1 stimulation of EphA2 is reported to lead to RhoA-dependent actomyosin contractility, which is in agreement with the observed cellular phenotypes in our experiments.Citation3,Citation26,Citation27 Interestingly, we found that the translocation of ligand-bound EphA2 followed that of the actomyosin contractility, thus suggesting a physical association between them. In order to identify the mechanism of actin reorganization and its connection with EphA2 transport, the Rho-kinase inhibitor Y27632 was used to block the cytoskeleton contraction pathway.Citation27 Analysis of the results revealed that the mechanism of ligand-receptor transport was mediated through a Rho-dependent pathway that actively transports EphA2 receptor clusters.
To elucidate the relation between EphA2 receptor motions and specific signaling cascades we used the “spatial mutation” strategy.Citation3,Citation5,Citation7,Citation8 In this approach, physical barriers fabricated onto the underlying substrate guide mobility of molecules in the supported membrane. These structures additionally impede the lateral motion of cell surface receptors through their action on bound ligands in the supported membrane. The technique is highly specific because only ligand-bound EphA2 receptors expressed on the cell surface are spatially reorganized. Interestingly, cells with spatially mutated EphA2 receptor organization showed: (a) altered f-actin morphology and (b) a decrease in the recruitment and the localization of the ADAM10 metalloprotease. Given the roles of secretases (such as ADAM10), and the cytoskeleton, the physical reorganization of the EphA2 receptor may have wide implications across multiple signaling pathways. ADAM10, for example, plays an important role in EphA receptor signaling since it takes part in ephrin ligand shedding; thus allowing for release of the physical tether between adjacent cells engaged in juxtacrine signaling. The spatial mutation strategy avoids off-target effects that are common when using pharmacological or genetic inhibition and thus it provided a direct link between receptor transport, ADAM10 recruitment and actin dynamics.
Why Mechanical Force? Biological Roles for Receptor Transport
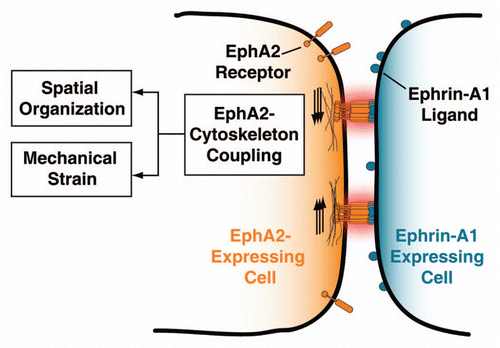
The direct consequences of EphA2 transport are two-fold (see ). The first is altering the size and the distribution of EphA2-ephrin-A1 clusters across the cell-cell junction. One may be tempted to draw parallels with the immunological synapse where the location of the T-cell receptor (TCR) can affect phosphorylation states.Citation5,Citation7,Citation8 This is not the case, and we did not observe clear differences in EphA2 phosphorylation as a function of receptor spatial organization.Citation3 The cellular mechanism of receptor transport may be similar, but signal outputs are different. A second consequence of EphA2 transport is the potential to apply mechanical strain on the EphA2-ephrin-A1 complex. If the ligand or the receptor encounters resistance to lateral transport, then the complex will experience tension and may undergo a conformational switch. The type of signaling mechanism (mechanotransduction model) is commonly cited for proteins involved in cellular adhesion such as the integrin family.Citation28–Citation31
An important question pertains to why the EphA2 pathway might incorporate sensitivity to force. The formation of complex tissues with controlled form and tensional homeostasis implies that cells can sense and react to very subtle changes in the mechanical properties of their environments.Citation28,Citation32,Citation33 In this regard, much attention has been focused on the integrins and associated focal adhesion proteins as master regulators of force.Citation31,Citation34 However, our work suggests that other receptors may incorporate sensitivity to force and we propose that mechanotransduction may be a common motif in signaling pathways. Mechanical aspects of the cellular microenvironment will change the spatial organization and the tension forces acting on all receptors whose ligands are surface associated. Correspondingly, it is likely that natural selection processes have explored this component of signal regulation.
In the case of EphA2, the increased ADAM10 recruitment found in the unrestricted EphA2 receptor clusters suggests that there may be enhanced levels of ligand cleavage and endocytosis of the ligand-receptor complex. The accepted mechanism for termination of ephrin forward signaling involves the regulated cleavage of ligands by the ADAM10 protease.Citation35,Citation36 Enhanced rates of EphA2 endocytosis would consume available ephrin-A1 ligand and may bias the inputresponse function of the entire system. Importantly, the EphA2 receptors interact with other signaling pathways, such as the chemokine receptors, integrins and cadherins,Citation37 which suggests that increased EphA2 receptor endocytosis may affect other signaling cascades.
Irrespective of the actual biological purpose for these behaviors, the experimental tools developed—the spatial mutation strategy—offer a route to uncovering mechanisms and signaling roles for receptor spatial organization. The technique enables single cell manipulations and facilitates quantitative characterization.Citation8 Importantly, this approach differs fundamentally from conventional tools employed for deconstructing the signaling roles of the cytoskeleton. For example, the most widely used approach consists of drug targeting of the cytoskeleton, which affects many signaling pathways and thus lacks specificity.
An open question to be addressed is the mechanism mediating the physical association of EphA2 to the cytoskeleton. The ERM family of intracellular proteins (which includes ezrin, radixin and moesin) are possible candidates since they are known to mediate dynamic binding between actin filaments and the cytoplasmic face of several transmembrane proteins.Citation38 ERM proteins are known to display diversity in their functions across different cell lines. For example, ezrin and moesin play an active role in the human T cell activation pathway by influencing the spatial organization of the immunological synapse.Citation1,Citation7,Citation17,Citation39 A multidisciplinary approach that combines advances in biophysical chemistry, optical microscopy/nanoscopy and cell biology will be required to identify and characterize the proteins mediating this coupling.
Given the mechanical sensitivity of the EphA2 pathway, it seems plausible that many other receptor pathways are susceptible to mechano-regulation. We speculate that receptor transport is a general mechanism used by a range of cells and receptors and, in different contexts, may be used to achieve specific goals. The advent of physical methods, such as the spatial mutation strategy, marks a clear path toward investigating the subtleties of mechanical/spatial transduction, and one that involves the confluence of biophysics, surface chemistry and cell biology.
Figures and Tables
Figure 1 Scheme depicting the mechanical coupling of ligand bound EphA2 clusters and the actin cytoskeleton. This physical coupling may alter the EphA2 pathway by: (i) changing the size and distribution of clusters, and (ii) imposing mechanical tension on the EphA2-ephrin-A1 complex. See text for details.
Addendum to:
References
- Hartman NaC, Nye JA, Groves JT. Cluster size regulates protein sorting in the immunological synapse. Proc Natl Acad Sci USA 2009; 106:12729 - 12734
- Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature 2010; 464:783 - 787
- Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science 2010; 327:1380 - 1385
- Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, et al. The immunological synapse balances T cell receptor signaling and degradation. Science 2003; 302:1218 - 1222
- Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science 2005; 310:1191 - 1193
- Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA 2007; 104:20296 - 20301
- DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. T Cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys J 2008; 94:3286 - 3292
- Mossman K, Groves J. Micropatterned supported membranes as tools for quantitative studies of the immunological synapse. Chem Soc Rev 2007; 36:46 - 54
- Manz BN, Groves JT. Spatial organization and signal transduction at intercellular junctions. Nat Rev Mol Cell Biol 2010; 11:342 - 352
- Klemm JD, Schreiber SL, Crabtree GR. Dimerization as a regulatory mechanism in signal transduction. Ann Rev Immunol 1998; 16:569 - 592
- Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 2002; 110:669 - 672
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000; 103:211 - 225
- Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell 1998; 94:277 - 280
- Gurry T, Endres RG. Biophysical mechanism for Ras-nanocluster formation and signaling in plasma membrane. PLoS ONE 2009; 4:6148
- Harding AS, Hancock JF. Using plasma membrane nanoclusters to build better signaling circuits. Trends Cell Biol 2008; 18:364 - 371
- Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: Lipid rafts and immune cell signaling. Ann Rev Immunol 2003; 21:457 - 481
- Qi SY, Groves JT, Chakraborty AK. Synaptic pattern formation during cellular recognition. Proc Natl Acad Sci USA 2001; 98:6548 - 6553
- Andrews NL, Lidke KA, Pfeiffer JR, Burns AR, Wilson BS, Oliver JM, et al. Actin restricts Fcepsilon RI diffusion and facilitates antigen-induced receptor immobilization. Nat Cell Biol 2008; 10:955 - 963
- Torres AJ, Vasudevan L, Holowka D, Baird BA. Focal adhesion proteins connect IgE receptors to the cytoskeleton as revealed by micropatterned ligand arrays. Proc Natl Acad Sci USA 2008; 105:17238 - 17244
- Martin KJ, Patrick DR, Bissell MJ, Fournier MV. Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS ONE 2008; 3:2994
- Zhuang G, Brantley-Sieders DM, Vaught D, Yu J, Xie L, Wells S, et al. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res 2010; 70:299 - 308
- Pasquale EB. Eph-Ephrin bidirectional signaling in physiology and disease. Cell 2008; 133:38 - 52
- Lackmann M, Boyd AW. Eph, a Protein Family Coming of Age: More Confusion, Insight or Complexity?. Sci Signal 2008; 1:2
- Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cellular Signalling 2004; 16:655 - 666
- Thanos CD, Goodwill KE, Bowie JU. Oligomeric structure of the human EphB2 receptor SAM domain. Science 1999; 283:833 - 836
- Fang WB, Ireton RC, Zhuang G, Takahashi T, Reynolds A, Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci 2008; 121:358 - 368
- Parri M, Buricchi F, Giannoni E, Grimaldi G, Mello T, Raugei G, et al. EphrinA1 activates a Src/Focal adhesion kinase-mediated motility response leading to Rho-dependent actino/myosin contractility. J Biol Chem 2007; 282:19619 - 19628
- Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 2009; 10:34 - 43
- Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci 2009; 122:179 - 186
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Revs Mol Cell Biol 2009; 10:63 - 73
- del Rio A, Perez-Jimenez R, Liu RC, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science 2009; 323:638 - 641
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009; 9:108 - 122
- Paszek MJ, Weaver VM. The tension mounts: Mechanics meets morphogenesis and malignancy. J Mamm Gland Biol Neopl 2004; 9:325 - 342
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat RevMol Cell Biol 2006; 7:265 - 275
- Mancia F, Shapiro L. ADAM and Eph: How ephrinsignaling cells become detached. Cell 2005; 123:185 - 187
- Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, et al. Adam meets Eph: An ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 2005; 123:291 - 304
- Arvanitis D, Davy A. Eph/ephrin signaling: networks. Gen Dev 2008; 22:416 - 429
- Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Molec Cell Biol 2010; 11:276 - 287
- Monks CRF, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998; 395:82 - 86