Abstract
The question of whether or not humans possess "free will" to control their own actions has preoccupied generations of scholars in the realm of Religion and Science. In Jewish theological literature, for instance, the contradiction between God's unlimited power of our destiny and Man's own "free will" represents one of the most challenging paradoxes of religious thought that is beyond our understanding. In Jewish philosophy, "Everything is foreseen; yet free will is given" (Rabbi Akiva, Pirkei Avoth 3:15), and this is where the paradox lies. But if we assume that humans do possess "free will" to make their own spontaneous choices, then this ability must be embedded in our brains. A crucial question then comes to mind: is "free will" unique to humans or is it a trait shared by other organisms as well?
The question of whether or not humans possess “free will” to control their own actions has preoccupied generations of scholars in the realms of Religion and Science. In Jewish theological literature, for instance, the contradiction between God's unlimited power of our destiny and Man's own “free will” represents one of the most challenging paradoxes of religious thought that is beyond our understanding. In Jewish philosophy, “Everything is foreseen; yet free will is given” (Rabbi Akiva, Pirkei Avoth 3:15), and this is where the paradox lies. But if we assume that humans do possess “free will” to make their own spontaneous choices, then this ability must be embedded in our brains. A crucial question then comes to mind: is “free will” unique to humans or is it a trait shared by other organisms as well?
Spontaneity in ‘Lower’ Organisms
Nonhuman animals, and especially ‘lower’ organisms such as invertebrates, are often seen merely as complex ‘automatons' that respond stereotypically to environmental cues. When scientists observe these animals responding in different ways to the same stimulus, such variations are usually attributed to “random errors in a complex brain”. But recently, there is accumulating evidence demonstrating that even ‘lower’ organisms are more than just automatons.Citation1–Citation4 For example, when fruit flies fly in a white and completely featureless arena, they express endogenously-created patterns of spontaneous behavior.Citation4 This suggests a nonrandom endogenous process of behavioral choice, which might imply a precursor motif of “free will”. The neuronal underpinnings responsible for generating this ‘rest state', however, remain elusive. The next logical step is thus to localize and understand the brain circuits responsible for such spontaneous behaviors.
In a recent study,Citation5 we present a unique and naturally-occurring phenomenon in which one insect uses neurotoxins to apparently ‘hijack the free will’ of another. Our investigation suggests one possible neuronal substrate involved in the regulation of spontaneous behavior in insects.
Jewel Wasps Manipulate Cockroach Behavior
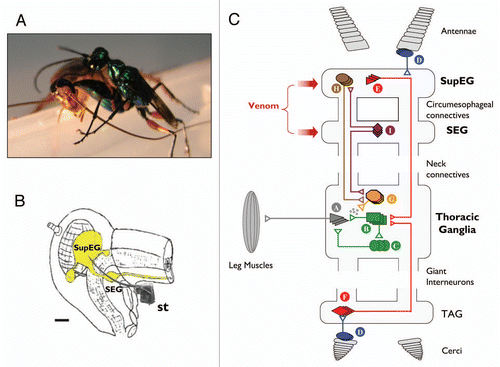
Cockroaches can fall victim to the parasitoid Jewel Wasp (Ampulex compressa), which employs them as live, yet immobile food supply for its larva. Unlike most other parasitoids, this tropical Ampulicine wasp does not simply paralyze its prey to immobilize it. Instead, it stings a cockroach in the head () and injects a neurotoxic venom cocktail directly inside the cerebral ganglia (). This turns the cockroach, metaphorically, into a submissive ‘zombie’: it gradually enters a long-lasting hypokinetic state, during which it becomes unresponsive to aversive stimuli and fails to self-initiate walking or escape behaviors. Although the stung cockroach is not paralyzed, it allows the wasp to cut both its antennae and drink hemolymph from the cut ends. The wasp then grabs one of the antennal stumps and pulls backwards, leading its prey into a pre-selected nest. The intoxicated cockroach, rather than fighting or fleeing its predator, actually follows the wasp submissively. In doing so it demonstrates a completely normal walking pattern, as if it was a dog led by his Master's leash. The wasp then lays one egg on the cockroach's leg, seals the nest and leaves the lethargic prey inside, still alive but powerless to escape under the influence of the venom. As the wasp larva hatches from the egg, it penetrates through the cockroach's cuticle and feeds on its internal organs for several more days. Only then, roughly five days after the sting, does the cockroach finally die and the larva pupates inside its abdomen, safe from predators outside the nest.
Behavioral and Physiological Aspects of Hypokinesia
The venom-induced hypokinetic state cannot be attributed to direct inhibition of muscles, motor neurons or sensory neurons, all of which are intact in stung cockroaches.Citation6,Citation7 Instead, we have found that the venom specifically elevates the threshold for the initiation and maintenance of walking-related behaviors, whereas other behaviors, e.g., righting, flying or grooming, are spared.Citation8 The unique symptoms of this ‘lethargic’ state can be illustrated by the following example: although stung cockroaches seldom express spontaneous or evoked walking under natural conditions, immersing them in water induces strong running-like movements, similar to those observed in immersed un-stung cockroaches. However, stung cockroaches maintain swimming for much shorter durations than un-stung cockroaches, as if they ‘despair’ faster.Citation8 This and other examples suggest that the venom selectively attenuates the ongoing ‘drive’ of cockroaches to produce walking-related behaviors, rather then their mechanical ability to do so.
The Neuronal Regulation of Behavioral Motivation
Which neuronal processes are modulated to attenuate the ‘drive’ for walking? Our recent data indicates that the behavioral manipulation of cockroaches is achieved by, at least, venom-induced inhibition of neuronal activity in the sub-esophageal ganglion (SEG). This small region in the cerebral CNS has been previously suggested to tonically regulate motor behavior through descending interneurons which, in turn, synapse onto thoracic premotor and motor circuitries ().Citation9–Citation12 We found that spontaneous and stimulus-evoked neuronal activity in the SEG is decreased in stung cockroaches, and that experimental injections of venom or local anesthetics into the SEG perturb walking initiation in normal cockroaches. In marked contrast, experimental injection of venom or anesthetics into a different part of the cerebral ganglia, namely the supraesophageal ganglion (SupEG), produces the opposite effect and promotes walking, even in the absence of external stimuli.Citation5,Citation13 These results are in agreement with previous lesions experiments (reviewed in ref. Citation12) and suggest that the motor pattern generator (PG) responsible for the expression of walking-related behaviors receive, simultaneously, tonic permissive inputs from the SEG and inhibitory inputs from the SupEG (). The same appears to be true for other PGs as well: the flying PG, for instance, was also found to receive tonic antagonistic inputs from the SEG and SupEG, although here the SEG inhibits the PG, while the SupEG is permissive.Citation12 Thus, the antagonistic interplay between descending inputs appears to regulate, either directly or indirectly through thoracic neuromodulatory neurons (such as the Dorsal or Ventral Unpaired Median neurons), the excitability of the different thoracic PGs. Internal and external sensory inputs, which represent different aspects of the animal in its environment, are thus expected to selectively modify the fine balance between permissive and inhibitory inputs descending to a specific PG, thereby priming the appropriate PG to favor the expression of one motor behavior over others. This, in turn, would determine the propensity of expression of different behaviors (such as walking or flying), and thus the ‘motivation’ of the animal to produce these behaviors. Several investigations suggest that the Central Body Complex and Mushroom Bodies, two distinct neuropiles in the SupEG which are involved in sensory integration and pre-motor processing,Citation14–Citation18 might also take part in the ongoing regulation of locomotion. Interestingly, when venom of the Jewel Wasp is traced in the cerebral ganglia of stung cockroaches, a large amount of venom is found in and around these SupEG neuropiles.Citation19 Hence, we are currently investigating the involvement of these neuropiles in the ‘zombification’ of cockroaches by the Jewel Wasp (reviewed in ref. Citation20).
To conclude, we hope that by investigating the neuronal basis of such parasite-induced alterations of host behavior, we might further our understanding of the neurobiology of the selection and initiation of behaviors and the associated neural mechanisms underlying changes in behavioral spontaneity. Our results indicate a mechanism which might cut through phylogenetic borders and could form the biological substrate for what we humans experience as “free will”.
Figures and Tables
Figure 1 (A) The parasitoid Jewel Wasp A. compressa stings its cockroach prey inside the head. (B) Schematic drawing of the cerebral nervous system (yellow) inside the cockroach's head capsule. the wasp's stinger (st., scanning electron micrograph drawn to scale) reaches to inject venom into both cerebral ganglia, namely the supra-esophageal ganglion (SupEG) and sub-esophageal ganglion (SEG). Scale bar: 0.5 mm. (C) Current model of the neurophysiological events leading to venom-induced hypokinesia in cockroaches stung by the Jewel Wasp. Schematic and simplified drawing of a cockroach nervous system, depicting circuitries that affect walking-related behaviors. The walking pattern generator that orchestrates leg movements is located in the thorax. It consists of motor neurons (A) innervating leg muscles, afferent neurons from sensory structures on the legs (not shown) and type-A thoracic interneurons (TIAs; B), which synapse onto the motor neurons directly and indirectly via local interneurons (C). The TIAs receive inputs from several interneurons. For example, sensory neurons (D) in the antennae or cerci recruit descending (E) or ascending (F) Giant Interneurons (GIs) in the SupEG and terminal abdominal ganglion (TAG), respectively. The GIs converge directly onto the TIAs to evoke escape responses. In addition, neurons of the pattern generator receive input from thoracic neuromodulatory cells (G). One example of these is the thoracic dorsal unpaired median (DUM) neurons, which secrete octopamine and modulate the efficacy of premotor-to-motor synapses. The neuromodulatory cells, in turn, receive tonic regulatory input from interneurons descending from the supra-esophageal ganglion (SupEG) (H) and sub-esophageal ganglion (SEG) (I). This tonic input affects the probability of occurrence of specific motor behaviors by modulating the different thoracic pattern generators directly (not shown) or indirectly (H and I). The wasp, A. compressa, injects its venom cocktail directly into both cerebral ganglia to modulate specific, yet unidentified cerebral circuitries. The current hypothesis states that in the SEG, the venom suppresses the activity of neuromodulatory neurons (I), presumably SEG-DUM neurons, which (1) ascend to the SupEG to regulate descending neuromodulatory (probably octopaminergic) neurons (H), and/or (2) descend to the thorax to regulate locomotory CPGs directly (not shown) or indirectly through thoracic neuromodulatory (probably DUM) neurons (G). Hence, the venom injected into the cerebral ganglia decreases the overall excitatory input to the thoracic walking pattern generator. As a result, walking-related behaviors are specifically inhibited and stimuli to the antennae or cerci fail to evoke normal escape responses. Figure modified from Libersat et al. (2009).Citation21
Addendum to:
References
- Greenspan RJ, Tononi G, Cirelli C, Shaw PJ. Sleep and the fruit fly. Trends Neurosci 2001; 24:142 - 145
- van Swinderen B. The remote roots of consciousness in fruit-fly selective attention?. Bioessays 2005; 27:321 - 330
- Menzel R, Brembs B, Giurfa M. Strausfeld NJ, Bullock TH. Cognition in Invertebrates. The Evolution of Nervous System 2006; 403 - 422
- Maye A, Hsieh CH, Sugihara G, Brembs B. Order in spontaneous behavior. PLoS ONE 2007; 2:443
- Gal R, Libersat F. A wasp manipulates neuronal activity in the sub-esophageal ganglion to decrease the drive for walking in its cockroach prey. PLoS ONE 2010; 5:10019
- Fouad K, Libersat F, Rathmayer W. The venom of the cockroach-hunting wasp Ampulex compressa changes motor thresholds: a novel tool for studying the neural control of arousal?. Zoology 1994; 98:23 - 34
- Fouad F, Libersat F, Rathmayer W. Neuromodulation of the escape behavior in the cockroach Periplaneta americana by the venom of the parasitic wasp Ampulex compressa. J Comp Physiol A 1996; 178:91 - 100
- Gal R, Libersat F. A parasitoid wasp manipulates the drive for walking of its cockroach prey. Curr Biol 2008; 18:877 - 882
- Kien J, Altman JS. Kien J, McCrohan CR, Winlow W. Decision-making in the insect nervous system: a model for selection and maintenance of motor programmes. Neurobiology of Motor Programme Selection 1992; 4:Oxford Pergamon Press 147 - 169 Pergamon Studies in Neuroscience
- Kien J, Altman JS. Preparation and execution of movement: parallels between insect and mammalian motor systems. Comp Biochem Physiol A 1992; 103:15 - 24
- Ridgel AL, Ritzmann RE. Effects of neck and circumesophageal connective lesions on posture and locomotion in the cockroach. J Comp Physiol A 2005; 191:559 - 573
- Gal R, Libersat F. New vistas on the initiation and maintenance of insect motor behaviors revealed by specific lesions of the head ganglia. J Comp Physiol A 2006; 192:1003 - 1020
- Libersat F, Haspel G, Casagrand J, Fouad K. Localization of the site of effect of a wasp's venom in the cockroach escape circuitry. J Comp Physiol A 1999; 184:333 - 345
- Martin J, Ernst R, Heisenberg M. Mushroom bodies suppress locomotor activity in Drosophila melanogaster. Learning Memory 1998; 5:179 - 191
- Martin JR, Raabe T, Heisenberg M. Central complex substructures are required for the maintenance of locomotor activity in Drosophila melanogaster. J Comp Physiol A 1999; 185:277 - 288
- Strauss R, Heisenberg M. A higher control center of locomotor behaviour in the Drosophila brain. J Neurosci 1993; 13:1852 - 1861
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol 2002; 12:633 - 638
- Wessnitzer J, Webb B. Multimodal sensory integration in insects—towards insect brain control architectures. Bioinsp Biomim 2006; 1:63 - 75
- Haspel G, Rosenberg LA, Libersat F. Direct injection of venom by a predatory wasp into cockroach brain. J Neurobiol 2003; 56:287 - 292
- Rosenberg LA, Glusman JG, Libersat F. Octopamine partially restores walking in hypokinetic cockroaches stung by the parasitoid wasp Ampulex compressa. J Exp Biol 2007; 210:4411 - 4417
- Libersat F, Delago A, Gal R. Manipulation of host behavior by parasitic insects and insect parasites. Annu Rev Entomol 2009; 54:189 - 207