Abstract
Exosomes are endosome-derived vesicles (40-100nm) formed during the formation of multi-vesicular bodies (MVBs). Occasionally, the MVBs fuse with the plasma membrane releasing their intra-luminal vesicles into the extracellular media, which are then known as exosomes. Different cell types such as B-cells, dendritic cells, platelets, reticulocytes and macrophages can release exosomes and current research in this area is more focused towards exosomes released by antigen-presenting cells. Exosomes have recently been shown to be immunomodulatory and the mechanism of immune response initiation by them is beginning to emerge. Besides molecules present inside the lumen of exosomes, it has been suggested that certain exosomal membrane molecules can interact with their surface receptors on the target cells thereby inducing an immunomodulatory response. In this review, Hsp70 and galectin-5, two immunogenic molecules present on exosomal membrane, are discussed in detail for initiating this response.
Exosomes are endosome-derived vesicles (40–100 nm) that are formed by the inward budding of the limiting membrane of the endosome resulting in the formation of multi-vesicular bodies (MVBs).Citation1 Under different physiological conditions, these MVBs fuse with the plasma membrane releasing the exosomes in the extracellular milieu.Citation1,Citation2 Exosomes have been shown to be released by variety of cells including APCs, monocytes, T-cells, reticulocytes, mast cells, tumor cells and platelets.Citation3–Citation9 They can be purified by differential centrifugation and floatation on a sucrose gradient with a density of 1.13–1.19 g/ml. Electron microscopy studies have revealed their typical ‘saucer-shaped’ morphology. In contrast to endosomes, the exosomes have inverse topology so that the extracellular domain of the protein is displayed on the outer surface of these vesicles.
Current research is focused towards deciphering the functional role of exosomes by characterizing their protein composition, which has been unveiled by WB, FACS and proteomics analysis.Citation3,Citation4,Citation10 These methods revealed that exosomes are composed of ubiquitous as well as cell-type specific proteins including components involved in antigen presentation (MHC class-I and class-II proteins), cytoskeletal proteins (actin and tubulin), membrane transport and fusion proteins (annexins and Rab proteins), tetraspanins (CD63, CD81, CD82, CD9 and CD86), integrins (a4b1, aMb2 and b2) and proteins belonging to the heat-shock family (Hsp70, Hsc70 and Hsp90).Citation3,Citation10 Originally observed as a mechanism to remove transferrin receptor during reticulocyte maturation,Citation11,Citation12 numerous studies have proposed the potential role of exosomes in initiation of immune activation.Citation13–Citation15 Here, I am discussing the recent studies on exosomal Hsp70 and galectin-5, which have provided new insights into the interaction between exosomes and immune response.
Exosomal Membrane Molecules and Immune Response Activation
Many studies have now suggested that exosomes can initiate an immunomodulatory activity. It was shown that higher amounts of exosomes are released after infection of macrophages with mycobacterial species M. smegmatis or M. avium.Citation13,Citation14 Interestingly, these exosomes activated a stronger pro-inflammatory response in the form of NF-⊠B activation and TNF⊠ release from untreated macrophages as compared to macrophages exposed to control exosomes. What makes exosomes pro-inflammatory? The molecular details of immune activation by exosomes are yet to be clearly demonstrated. One possible explanation provided is that these exosomes contain mycobacterial cell wall components that act as PAMPs in activating the immune system.Citation14 However, exosomes isolated from tumor cells have also been shown to be immunomodulatory, which suggests that exosomes may contain molecules, other than bacterial PAMPs, that can induce an immune response. Therefore, a more objective explanation is that these two different conditions, tumor progression and bacterial infection, use a combination of host molecules and disease-specific molecules/PAMPs/ DAMPs that act mutually when it comes to alert and activation of the immune system.
One of the major host components in exosomes is Hsp70. It is a molecular chaperone belonging to the heat-shock family of proteins and is mainly localized in the cytosol.Citation16 In our studies, we observed that a fraction of this protein is detected on exosomal membrane after mycobacterial infection of macrophagesCitation13 or heat-shock treatment at 43°C (). The total pool of Hsp70 in the exosomes decreased after surface trypsinization suggesting both cytosolic as well as membrane-association of Hsp70.Citation13 In accordance with the findings with exosomes, a similar set of pro-inflammatory parameters, TNF⊠ and NF-⊠B, was also simulated after treatment of macrophages with rHsp70 (recombinant Hsp70). Furthermore, rHsp70 treatment also increased phagocytosis and maturation of latex-bead phagosomes.Citation13 How does Hsp70 increase phagocytosis? What are the innate immune receptors that it can interact with? Previous studies have demonstrated that rHsp70 can bind to different TLRs on the cell surface leading to activation of NF-⊠B and MAP kinase pathways.Citation17–Citation19 Stimulation of TLRs, in general, leads to increased pro-inflammatory response including phagocytosis.Citation20,Citation21 Therefore, it is possible that exosomal Hsp70 interacts with TLRs on the cell surface thereby initiating signaling pathways, such as NF-⊠B (proposed model depicted in ), that ultimately leads to increased phagocytosis and phago-lysosome fusion. Increase in phagocytosis of yeast particles with rHsp70 treatment has also been shown to initiate specifically through TLR7 in competitive experiments where phagocytosis induced by rHsp70 was blocked when cells were first exposed to different TLR7 ligands.Citation22 Although this study demonstrated a role for TLR7, it is likely that exosomal Hsp70 may as well bind to other TLRs as reported previously for rHsp70 by some studies.Citation18,Citation19,Citation23,Citation24 These contradictions might arise due to the possible differences in confirmation of exosomal and purified Hsp70 as well as due to differences in the source and preparation of recombinant Hsp70 used in various studies.
Recent study by Chalmin et al. (2010) also highlighted the role of exosomal Hsp70 in tumor biology.Citation25 The authors observed that exosomes isolated from different mouse tumor cell lines displayed Hsp70 (Hsp72 in the original paper) on their surface. Further, rHsp70 triggered pStat3 expression and IL-6 production in myeloid-derived suppressor cells (MDSCs) obtained from wild-type mice but not from TLR2-deficient mice.Citation25 This response was blocked by treatment with either anti-Hsp70 antibody or anti-IL-6 antibody demonstrating that TLR-2 dependent IL-6/Stat3 pathway is involved. Besides being a chaperone, the cytokine properties of Hsp70 are also well documented in studies showing its interaction with TLR4/CD14 complexesCitation18,Citation23,Citation26 or to TLR2.Citation23,Citation27 Similarly, studies by Multhoff's group showed that tumor cells that express higher Hsp70 on their surface are killed significantly better by natural-killer (NK) cells compared to tumor cells that express low Hsp70 levels.Citation28,Citation29 Only exosomes with membrane Hsp70 (released by Hsp70-positive tumor cells) stimulated migration of NK cells that displayed increased lytic activity against tumor cells through granzyme B release.Citation28 This finding is also supported by another study that showed activation of mouse NK cells by rHsp70.Citation30 The above-mentioned studies offer enough evidence emphasizing immune system activation by exosomal Hsp70.
Another interesting molecule, galectin-5, has also been detected within exosomes.Citation31 Galectin is a type of lectin that shows affinity for ⊠-galactoside. Extracellular gelectins cross-link cell surface and extracellular glycoproteins and may thereby modulate cell adhesion and induce intracellular signals.Citation32 Mainly cytosolic, a fraction of galectin-5 was also observed on the cell surface of reticulocytes and erythrocytes from where it was secreted in association with exosomes partly on the exosomal membrane.Citation31 It was observed that galectin-5 positive exosomes were phagocytosed into rat peritoneal macrophages and J774 macrophages that showed decrease in their uptake when cells were first treated with purified galectin-5 demonstrating that exosome uptake is galectin-5 dependent.Citation31 Surface presence of other members of galectin family is also not ruled out. Indeed galectin-9 has also been shown to be present on exosome surface.Citation33
Conclusions and Perspective
The immunological significance of exosomes is still far from clear. However, many studies have reported their potential to activate the immune system, which might be their major function. Consistent with this, exosome release is enhanced in critical conditions where immune activation is required. One explanation, supported by recent studies,Citation13,Citation25,Citation31 suggests the presence of immunogenic molecules itself on the exosomal membrane for immune system activation. Favoring this idea is the fact that the topology of the molecules displayed on exosomes is analogous to that observed on plasma membrane. This makes them well suitable for interaction with their cell surface receptors thereby mediating signal transduction without the need for two cells to be in direct contact. Further, these vesicles can also fuse with the recipient cell leading to acquisition of novel molecules by the cells as has already been reported for the delivery of mRNA and miRNA through this route.Citation34 However, there are still number of open questions: What signal enhances exosome release? How do exosomes fuse to the target cells? Clearly more studies are required to answer these questions. Future work together with new insights will elucidate these mechanisms that can be applied for the development of exosomes as novel therapeutic vaccines. The scientific and clinical implications of research in this area will be very noteworthy and an interesting facet of exosome biology in the coming years.
Abbreviations
| TLRs | = | toll-like receptors |
| MVBs | = | multi-vesicular bodies |
| PAMPs | = | pathogen-associated molecular patterns |
| DAMPs | = | danger-associated molecular patterns |
| Hsp | = | heat-shock protein |
| APCs | = | antigen presenting cells |
Figures and Tables
Figure 1 EM image showing presence of Hsp70 on exosome membrane. RAW 264.7 mouse macrophages were given heat-shock at 43°C for 2 h. After 6 h recovery at 37°C, exosomes were purified from culture supernatants by differential centrifugation and floatation on a sucrose gradient. The samples were processed for surface immunogold labeling with anti-Hsp70 antibody. Arrows show Hsp70 (gold) labeling on exosome surface. Insets show magnified images of exosomes with surface Hsp70 labeling. (Micrograph provided by Christopher K.E. Bleck).
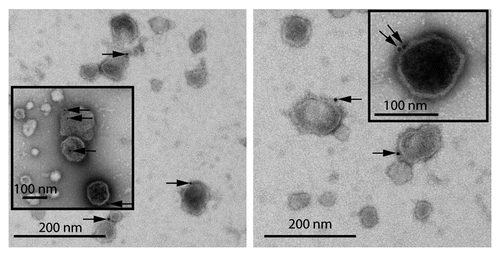
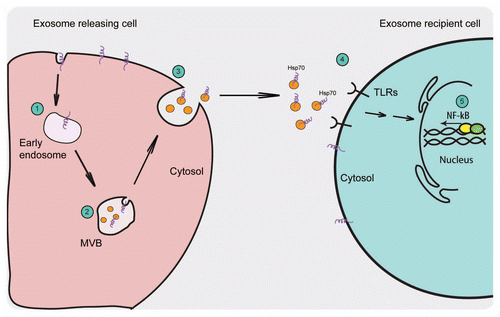
Figure 2 Schematic representation of the exosome release pathway and the proposed activation of the immune response by exosomal Hsp70. (1) Extracellular molecules or membrane proteins are internalized into the endosomes that form intra-luminal vesicles by the inward budding of their limiting membrane. (2) Under certain conditions, these MVBs fuse with the plasma membrane releasing their ILVs to the outside where they are known as exosomes. (3) Exosomes exhibit the same orientation of their membrane proteins as on the plasma membrane with extracellular domain of the protein present on the exosomal surface. (4) Exosomes that are Hsp70-positive can interact with TLRs on the cell surface (5) thus activating NF-⊠B signaling pathway.
Acknowledgements
The author would like to thank Gareth Griffiths (Institute for Molecular Biosciences, Oslo, Norway) for helpful discussions; David Liebl (Institute for Molecular Bioscience, Brisbane, Australia) and A.A. Jeyaprakash (Max-Planck Institute, Martinsried, Germany) for critical reading of the manuscript, and Christopher K.E. Bleck (C-CINA, University of Basel, Basel, Switzerland) for providing electron micrograph in . This work was supported by Alexander von Humboldt Foundation, Germany and European Union Sixth Framework Program.
References
- Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 2004; 16:415 - 421
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000; 113:3365 - 3374
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 2001; 166:7309 - 7318
- Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 2003; 278:10963 - 10972
- Dardalhon V, Geminard C, Reggio H, Vidal M, Sainte-Marie J. Fractionation analysis of the endosomal compartment during rat reticulocyte maturation. Cell Biol Int 2002; 26:669 - 678
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999; 94:3791 - 3799
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183:1161 - 1172
- Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 2003; 170:3037 - 3045
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL crosspriming. Nat Med 2001; 7:297 - 303
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2:569 - 579
- Blanc L, Vidal M. Reticulocyte membrane remodeling: contribution of the exosome pathway. Curr Opin Hematol 2010; 17:177 - 183
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983; 33:967 - 978
- Anand PK, Anand E, Bleck CK, Anes E, Griffiths G. Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PLoS One 2010; 5:10136
- Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem 2007; 282:25779 - 25789
- Schartz NE, Chaput N, Andre F, Zitvogel L. From the antigen-presenting cell to the antigen-presenting vesicle: the exosomes. Curr Opin Mol Ther 2002; 4:372 - 381
- Multhoff G. Heat shock protein 70 (Hsp70): membrane location, export and immunological relevance. Methods 2007; 43:229 - 237
- Asea A. Heat shock proteins and toll-like receptors. Handb Exp Pharmacol 2008; 111 - 127
- Chase MA, Wheeler DS, Lierl KM, Hughes VS, Wong HR, Page K. Hsp72 induces inflammation and regulates cytokine production in airway epithelium through a TLR4- and NFkappaB-dependent mechanism. J Immunol 2007; 179:6318 - 6324
- Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol 2002; 270:169 - 184
- Blander JM. Phagocytosis and antigen presentation: a partnership initiated by Toll-like receptors. Ann Rheum Dis 2008; 67:44 - 49
- Underhill DM, Gantner B. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect 2004; 6:1368 - 1373
- Wang R, Town T, Gokarn V, Flavell RA, Chandawarkar RY. HSP70 enhances macrophage phagocytosis by interaction with lipid raft-associated TLR-7 and upregulating p38 MAPK and PI3K pathways. J Surg Res 2006; 136:58 - 69
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 2002; 277:15028 - 15034
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 2002; 277:15107 - 15112
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010; 120:457 - 471
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 2000; 6:435 - 442
- Asea A. Chaperokine-induced signal transduction pathways. Exerc Immunol Rev 2003; 9:25 - 33
- Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 2005; 65:5238 - 5247
- Multhoff G. Activation of natural killer (NK) cells by heat shock protein 70. Int J Hyperthermia 2009; 25:176 - 179
- Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeboller H, et al. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol 2007; 179:5523 - 5533
- Barres C, Blanc L, Bette-Bobillo P, Andre S, Mamoun R, Gabius HJ, et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood 2010; 115:696 - 705
- Cummings R, Liu F. Galectins 2009; Cold Spring Harbor (NY) Cold Spring Harbor Laboratory Press
- Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 2009; 113:1957 - 1966
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9:654 - 659