Abstract
G protein-coupled receptors (GPCRs) are subject to the regulation by protein kinases. By controlling the phosphorylation-dephosphorylation balance, protein kinases actively modify GPCR expression and function. In a recent study, we have identified a novel phosphorylation-dependent regulation of Gαi/o-coupled muscarinic acetylcholine receptors. A synapse-enriched protein kinase, Ca2+/calmodulin-dependent protein kinase II (CaMKIIα), binds directly and selectively to second intracellular loops of muscarinic M4 receptors (M4Rs). This Ca2+-sensitive binding enables CaMKIIα to phosphorylate M4Rs at a selective threonine residue. In rat striatal neurons which abundantly express M4Rs, rapid cytoplasmic Ca2+ rises enhance the association of CaMKIIα with M4Rs and increase threonine phosphorylation of the receptor. This CaMKIIα-mediated phosphorylation results in a potentiation of M4R activity, which is critical for controlling cellular and behavioral responsivity to dopamine stimulation. In sum, our data identify a novel kinase-GPCR interaction. Through a Ca2+/activity-sensitive manner, CaMKIIα contributes to maintaining acetylcholine-dopamine homeostasis in the basal ganglia.
G protein-coupled receptors (GPCRs) are regulated by various cytoplasmic protein kinases through a mechanism involving protein-protein interactions and/or phosphorylation. The serine/threonine protein kinase, Ca2+/calmodulin-dependent protein kinase II (CaMKII), is one of those kinases that actively regulate GPCRs via phosphorylation. CaMKII is abundant in brain cells and is enriched at synaptic sites.Citation1 It becomes activated after Ca2+ and calmodulin (CaM) bind to the kinase. Once activated, CaMKII accesses and phosphorylates various substrates. Meanwhile, CaMKII induces autophosphorylation, which renders the enzyme a Ca2+/CaM-independent (autonomous) activity even after the initial Ca2+ stimulus subsides.Citation2–Citation4 CaMKII is believed to interact with and regulate many targets,Citation5 although a limited number of them have been identified so far. In searching for a new target, muscarinic acetylcholine receptors (mAChRs) have drawn our attention. mAChRs are members of the GPCR superfamily. Five mAChR subtypes (M1–M5) are coupled to either Gαq (M1, M3 and M5) or Gai/o (M2 and M4) proteins.Citation6,Citation7 Since receptors are densely expressed in broad regions of mammalian brains,Citation8 they regulate various cellular and synaptic activities in many brain circuits. Malfunction of these receptors is also linked to the pathogenesis of some common psychiatric and neurodegenerative disorders.Citation9,Citation10
In our recent study, we identified M4 receptors (M4Rs) as a new interacting partner of CaMKII.Citation11 In a series of in vitro protein-protein interaction assays, we found that CaMKIIα bound directly to one of intracellular domains of the receptor. Unexpectedly, CaMKIIα bound to a relatively small intracellular domain, the second intracellular loop (M4RIL2), rather than the large third intracellular loop. The interaction between the two proteins is noticeably selective based on findings that (1) CaMKIIα, but not CaMKIV, bound to M4Rs and (2) among second loops from all five muscarinic subtypes, CaMKIIα bound to only M4RIL2. In addition, the affinity of CaMKIIα for M4RIL2 largely relies on activation of the kinase by Ca2+/CaM, a feature seen in many targets.Citation12 T286-autophosphorylation also impacts on binding properties of the kinase. Generally, autophosphorylation enhances binding of the kinase to a given interacting partner. This is indeed the case for M4Rs.
Biochemical binding of CaMKIIα to M4RIL2 may or may not lead the kinase to phosphorylate M4RIL2. Sequence analysis of the CaMKIIα-binding site in M4RIL2 reveals a consensus substrate recognition motif (RXXT/S) for CaMKII.Citation13 This, together with the finding that the catalytic domain is the part of the kinase binding to M4RIL2, suggests M4RIL2 to be a potential phosphorylation substrate of the kinase. In fact, evidence shows that M4RIL2 serves as a preferred substrate of CaMKIIα. An accurate phosphorylation site (Thr145) resides within the consensus phosphorylation motif of M4RIL2.
The in vitro interaction between recombinant CaMKII and M4Rs needs to be validated for native proteins in vivo. In rodent brains, M4Rs are most abundantly present in the striatum and are preferentially localized at postsynaptic sites.Citation14–Citation16 We thus targeted the striatum to define the protein-protein interaction between endogenous CaMKIIα and M4Rs in vivo. In a complete set of coimmunoprecipitation experiments, physical interactions between the two partners in striatal neurons were established. This interaction and its specificity were confirmed by the lack of the interaction in M4R-deficient mice. Interestingly, in vivo CaMKIIα-M4R interactions are subject to a positive regulation by Ca2+. This important property indicates a previously unrecognized Ca2+ route linking CaMKII to M4Rs in an activity-dependent manner. Nevertheless, coimmunoprecipitation data provide no clear indication about whether interacting proteins interact with each other directly or indirectly. Due to this reason, we expanded our study to include experiments more directly towards solving this issue. Based on the core CaMKIIα-binding sequence on M4RIL2 (YPARRTTKM) we identified, we developed an interaction-interfering peptide (M4Ri). This interaction-dead peptide is rendered cell-permeability in living neurons by fusing a Tat transduction domain and can competitively and selectively inhibit the CaMKII binding to a specific site of M4Rs, i.e., M4RIL2. With this peptide, we demonstrated an in vivo model in striatal neurons, in which Ca2+ rises directly recruited CaMKIIα to an M4Ri-sensitive site on M4RIL2.This induced a CaMKII-mediated and CaMKII-M4RIL2 interaction-dependent phosphorylation of M4Rs. Of note, an actual level of phosphorylation at the specific Thr145 site has not been examined. A site- and phosphospecific antibody or a proteomic tandem mass spectrometry analysis is needed, if feasible, to portray accurate responses of M4RIL2 phosphorylation at Thr145 to Ca2+ signals.
Kinase-triggered phosphorylation provides effective posttranslational modifications of protein expression and function. CaMKII by phosphorylating M4RIL2 may have a significant impact on M4R signaling. Gαi/o-coupled M4Rs principally inhibit adenylyl cyclase, thereby lowering the rate of cAMP production.Citation6,Citation7 By measuring changes in cAMP levels, we established a sequential model in striatal neurons. A rapid rise in cytoplasmic Ca2+ levels activates CaMKII. Active CaMKII then associates with M4Rs and thereby potentiates M4R efficacy in suppressing cAMP production. Ca2+ sources to initiate these events have not been explored in our study.Citation11 Potential sources include, but not limited to, (1) ligand-gated Ca2+ channels such as glutamate NMDA receptors, (2) Gaq-coupled group I metabotropic glutamate receptors (mGluR1/5) which release Ca2+ from intracellular stores via a phospholipase Cβ (PLCβ)-dependent mechanism, (3) voltage-operated Ca2+ channels and/or (4) even Gaq-coupled D1 receptors that increase intracellular Ca2+ release via the PLCβ pathway.Citation17,Citation18 Of note, M4Rs have been found in the postsynaptic density (PSD) of asymmetrical (excitatory) synapses in the rat striatum in an ultrastructural analysis.Citation16 It is then possible that Ca2+ rises through either ionotropic or metabotropic glutamate receptors may affect M4Rs in the PSD microdomain via CaMKII. This may link glutamatergic, cholinergic and adjacent dopaminergic receptors into an effective signaling machinery controlling cellular and synaptic activity.
In addition to acetylcholine, dopamine is another prime neurotransmitter in the basal ganglia. The dopamine-acetylcholine balance is one of most important interactions in this region and is critical for maintaining proper limbic homeostasis and for the pathogenesis of neurological disorders derived from either dopamine overload, such as addictive behaviors to psychostimulants or dopamine depletion, such as Parkinson's disease. At the cellular level, both M4Rs and dopamine D1 receptors are preferentially co-expressed in striatonigral projection neurons,Citation19,Citation20 which strategically constitutes a cellular template for operating the dopamine-acetylcholine balance. Given an active role of CaMKII in regulating striatal M4Rs, the kinase may serve well as an essential regulator of the dopamine-acetylcholine integration. Indeed, CaMKII synergizes with M4Rs to balance increased D1 activity. Under a stimulated state in response to a D1 agonist or the psychostimulant cocaine which increases synaptic dopamine levels by blocking reuptake of released dopamine, the D1 receptor tone is enhanced to activate adenylyl cyclase and increase cAMP formation in D1-bearing neurons. At these same neurons, the M4R efficacy in inhibiting adenylyl cyclase and cAMP formation is potentiated simultaneously by CaMKII. This provides a Ca2+/activity-dependent mechanism to prevent overstimulation of D1 signaling or to bring in a heterologous desensitization of D1 receptor responses. Behaviorally, the CaMKII-regulated dopamine-acetylcholine balance controls movement. By balancing D1 and M4R activity in striatonigral efferent neurons, the kinase participates in the regulation of the ‘direct pathway’ outflow and motor activity. In the case of cocaine stimulation, elevated dopamine activates D1 receptors on striatonigral neurons to increase cAMP levels and activate downstream protein kinase A, which in turn upregulates excitability of these neurons and stimulates motor activity.Citation21 Similar to dopamine, acetylcholine responds to cocaine by showing an increased local release.Citation22,Citation23 Cellular CaMKII activity in striatonigral neurons may also be enhanced by a Ca2+ entry to be identified. Through both increased extracellular acetylcholine levels and augmented intracellular CaMKII activity, M4R efficacy is upregulated to match an enhanced D1 activity. This ultimately contributes to restricting motor responses to cocaine and balancing an unbalanced dopamine-acetylcholine level. Given the fact that the dopamine-acetylcholine system is linked to the pathogenesis of psychiatric disorders such as addiction and neurodegenerative disorders such as Parkinson's disease, future interesting studies will be needed to elucidate the accurate role of the CaMKII-M4R coupling in the progressive development of these disorders.
Figures and Tables
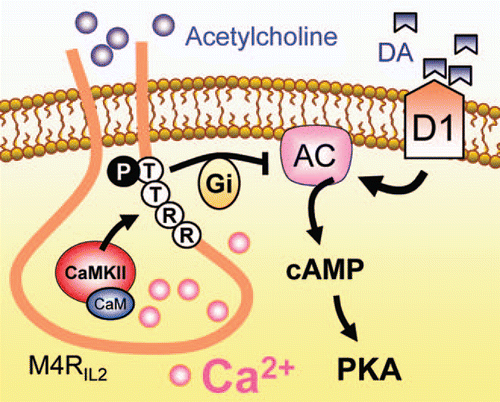
Figure 1 A model of CamKII-M4R interactions linking Ca2+ to M4Rs. Synergistic activation of M4Rs by an intracellular mechanism involving the Ca2+-regulated CamKII association with M4Rs potentiates the efficacy of M4Rs in inhibiting cAMP signaling. Ca2+ activates CaMKII to increase its binding to the C-terminal region of the second intracellular loop of M4Rs (M4RIL2). This enhances the inhibitory tone of M4Rs on cAMP responses to dopamine. Other abbreviations: AC, adenylyl cyclase; CaM, calmodulin; DA, dopamine; PKA, cAMP-dependent protein kinase A.
Acknowledgements
This work was supported by NIH grants DA10355 (J.Q.W.) and MH61469 (J.Q.W.) and by a grant from Saint Luke's Hospital Foundation.
Addendum to:
References
- Kelly PT, McGuinness TL, Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA 1984; 81:945 - 949
- Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 2002; 71:473 - 510
- Colbran RJ, Brown AM. Calcium/calmodulindependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol 2004; 14:318 - 327
- Griffith LC. Regulation of calcium/calmodulindependent protein kinase II activation by intramolecular and intermolecular interactions. J Neurosci 2004; 24:8394 - 8398
- Colbran RJ. Targeting of calcium/calmodulindependent protein kinase II. Biochem J 2004; 378:1 - 16
- Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol 1996; 10:69 - 99
- Nathanson NM. A multiplicity of muscarinic mechanism: Enough signaling pathways to take your breath away. Proc Natl Acad Sci USA 2000; 97:6245 - 6247
- Nathanson NM. Synthesis, trafficking and localization of muscarinic acetylcholine receptors. Pharmacol Ther 2008; 119:33 - 43
- Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther 2008; 117:232 - 243
- Scarr E, Dean B. Muscarinic receptors: do they have a role in the pathology and treatment of schizophrenia?. J Neurochem 2008; 107:1188 - 1195
- Guo ML, Fibuch EE, Liu XY, Choe ES, Buch S, Mao LM, et al. CaMKIIα interacts with M4 muscarinic receptors to control receptor and psychomotor function. EMBO J 2010; 29:2070 - 2081
- Liu XY, Mao LM, Zhang GC, Papasian CJ, Fibuch EE, Lan HX, et al. Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron 2009; 61:425 - 438
- White RR, Kwon YG, Taing M, Lawrence DS, Edelman AM. Definition of optimal substrate recognition motifs of Ca2+-calmodulin-dependent protein kinases IV and II reveals shared and distinctive features. J Biol Chem 1998; 273:3166 - 3179
- Levey AL, Kitt CA, Simonda WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtypespecific antibodies. J Neurosci 1991; 11:3218 - 3226
- Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, et al. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: distribution of m4 and m5 receptors in rat brain. Mol Pharmacol 1993; 43:149 - 157
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci 1994; 14:3351 - 3363
- Felder CC, Jose PA, Axelrod J. The dopamine-1 agonist, SKF82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther 1989; 248:171 - 175
- Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther 1990; 253:987 - 992
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse 1997; 27:357 - 366
- Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res 2001; 894:12 - 20
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 2007; 30:228 - 235
- Imperato A, Obinu MC, Gessa GL. Effects of cocaine and amphetamine on acetylcholine release in the hippocampus and caudate nucleus. Eur J Pharmacol 1993; 238:377 - 381
- Zocchi A, Pert A. Alterations in striatal acetylcholine overflow by cocaine, morphine and MK-801: relationship to locomotor output. Psychopharmacology 1994; 115:297 - 304