Abstract
In humans, there is an interdependent relationship between aging and immune system function, with each process affecting the outcome of the other. Aging can trigger immune system dysfunction, and alterations in the immune response can in turn affect human lifespan. Genetic experiments in model organisms such as C. elegans and Drosophila have led to the identification of numerous genes and signaling pathways that can modulate organismal lifespan and immune system function. Importantly, many of these signaling pathways exhibit conserved function in multiple species, including mammals, suggesting that the research in these simpler models could one day pave the way for the modulation of aging and immunity in humans. Here, we review the recent progress in our understanding of aging, innate immunity, and the interaction between these two processes using these simple model systems. Additionally, we discuss what this may tell us about aging and the innate immune system in humans.
Aging and Immunity
In humans, the relationship between aging and the immune response is a complicated one. Aging is characterized by an overall decline in immune function termed immunosenescence, which affects both the innate and adaptive immune systems.Citation1,Citation2 With age, cells of the innate immune system exhibit decreased function; for example, macrophages and neutrophils exhibit a weaker phagocytic response and a weaker oxidative burst. Dendritic cells from older individuals have a decreased ability to stimulate T cells. Cells of the adaptive immune system are also affected by age. T cells in older individuals display decreased T cell memory. There is also a decrease in the naïve T cell population in the thymus and decreased B cell production present in the elderly. Although older individuals have a weaker immune response, conversely, they also display a chronic inflammatory state termed inflammaging,Citation3 characterized by an increase in inflammatory cytokines present in the serum and an increase in the total NK cell count.Citation1,Citation2 Immunosenescence leads to a decreased vaccine response and an increased risk of infection; inflamm-aging could contribute to a host of inflammatory diseases such as atherosclerosis. Thus, aging can have dramatic effects on the immune response, and conversely, the immune system can affect the risk of many ageassociated diseases and can therefore affect lifespan. Thus, understanding the relationship between aging and immune system function is of critical importance to human health, particularly as the average human lifespan lengthens, increasing the impact of age-related diseases.
Model Systems to the Rescue: The Genetics of Lifespan Regulation
The quest to understand aging and age-related diseases has been a long and ongoing one, with much of the research focusing on environmental damage and the organism's response to that damage. However, recent genetic experiments in model organisms such as C. elegans, Drosophila and mice have led to something of a revolution in aging research, as conserved signaling pathways that affect lifespan have been identified. Interestingly, the pathways that modulate lifespan also regulate the response to various environmental stresses, ranging from oxidative damage to UV exposure to thermal stress. Several of these signaling pathways also regulate the immune response. The genetic tools available in these model systems allow the dissection of the interaction between aging, immunity and stress response.
Much of this revolution in aging research has originated with studies using the nematode C. elegans. C. elegans was originally developed by Sydney Brenner as a model system to study development and nervous system function,Citation4 but it has emerged as a leading model system in many fields, including lifespan research.Citation5 This lifespan research has been aided by the excellent genetic and genomic tools available to study C. elegans, as well as the relatively short nematode lifespan (2–3 weeks). The modern explosion of aging research in C. elegans started when mutations were identified in genes in the insulin/IGF-1 (insulin-like growth factor 1)-like signaling pathway that affect nematode lifespan. Mutation of either daf-2 (the nematode homolog of the insulin/IGF-1 receptor) or age-1 [a phosphatidylinositol 3-kinase (PI3K) that acts in the daf-2 pathway] was shown to lead to drastically increased nematode lifespan,Citation6–Citation8 a concomitant increase in nematode health and resistance to many environmental stresses, including oxidative stress, ultraviolet light, heavy metals and thermal stress.Citation9–Citation17 Mutation of either daf-2 or age-1 also increases C. elegans DNA repair capacity.Citation13 Finally, mutation of either daf-2 or age-1 affects the nematode immune response; daf-2 and age-1 mutant nematodes survive longer than wild-type nematodes when exposed to the human bacterial pathogens P. aeruginosa, S. aureus and E. faecalis.Citation18
Since the original demonstration that the insulin/IGF-1 signaling pathway modulates nematode lifespan, numerous other genes, pathways and treatments have been discovered that affect C. elegans lifespan, including the action of the nematode germline, mitochondrial activity, caloric restriction and many others (reviewed recently in Kenyon 2010 Citation5). Ablation of the nematode germline, either physically using a laser to ablate the germline precursor cells or genetically using sterilizing mutations, leads to increased nematode lifespanCitation19,Citation20 and resistance to oxidative and thermal stress.Citation19 The germline has also been found to regulate C. elegans immunity, as nematodes lacking a germline are resistant to the pathogens P. aeruginosa, S. marsescens and S. aureus.Citation21–Citation23 The relationship between lifespan regulation and innate immunity is not a universal one in C. elegans, however, because some mutations that increase nematode longevity (such as clk-1, a regulator of ubiquinone symthesis and eat-2, which is often used as a model for caloric restriction) do not increase nematode pathogen resistance.Citation22 So far, only the insulin/IGF-1 pathway and the germline pathways have been shown to regulate both C. elegans lifespan and immunity, so we will focus on these two pathways and discuss how their roles in both processes are interrelated.
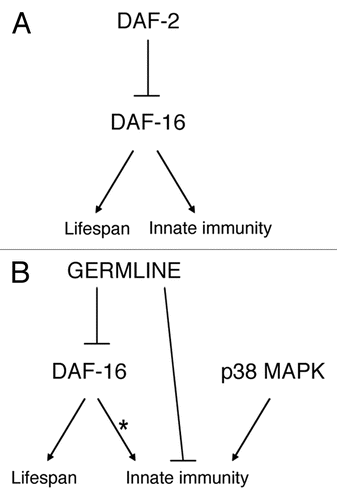
Numerous components of the insulin/IGF-1 pathway have been identified in C. elegans.Citation5,Citation24–Citation27 daf-2 is the sole insulin/IGF-1-like receptor in C. elegansCitation28 although numerous insulin-like peptides are encoded in the genome.Citation29,Citation30 daf-2 acts by the activation of the PI3 Kinase, age-1,Citation31 which in turn acts through the downstream kinases pdk-1, akt-1, akt-2 and sgk-1 ().Citation32–Citation34 The actions of these kinases culminate in the phosphorylation and inactivation of a FOXO-family transcription factor, DAF-16, which is sequestered from the nucleus when inactive.Citation35–Citation38 However, when daf-2 signaling is reduced, daf-16 can undergo nuclear translocation,Citation37 where in conjunction with other transcription factors, it initiates the transcription of numerous genes that affect stress and pathogen resistance.Citation39,Citation40 Thus, daf-16 is a critical readout of insulin signaling that regulates lifespan, stress resistance and pathogen resistance. The insulin/IGF-1 pathway is also modulated by the C. elegans PTEN homolog daf-18.Citation41
The C. elegans germline can also regulate nematode lifespan. Removal of the germline leads to increased lifespan.Citation19,Citation20 This effect is due to germline proliferation and not simply loss of progeny production, as removal of the entire gonad (germline and somatic tissues) does not lengthen lifespan.Citation20 The germline acts through the adaptor protein kri-1 to ultimately control DAF-16 activation.Citation42 Like the effect of daf-2 on lifespan, the effect of the germline also requires the daf-16 transcription factor ().Citation19,Citation20
The C. elegans Innate Immune Response
C. elegans lacks an adaptive immune response and also lacks obvious migratory immune cells (the possible exception to this may be the coelomocytes, which are endocytic migratory cells in the nematode, although no immunological role for these cells has been demonstratedCitation43). Instead, the main route of host defense in C. elegans appears to be the production of a plethora of antimicrobial proteins that are expressed in tissues exposed to pathogens.Citation44–Citation50C. elegans can be infected by numerous Gram negative and positive bacteria and some fungi.Citation51 While there are exceptions, the majority of these pathogens infect the nematode intestine, proliferate there and ultimately kill the nematodes.Citation51 The production of different subsets of these antimicrobial proteins is induced by different pathogens, with pathogen-specific signatures suggesting that there are multiple pathogen recognition receptors in the nematode.Citation44–Citation50
Several genes and pathways have been identified that affect production of these antimicrobials in response to pathogen and therefore, ultimately affect the survival of the infected nematode. These include but are not limited to a p38 MAP Kinase signaling pathway,Citation45,Citation46,Citation52 a TGFβ signaling pathway,Citation47,Citation53 and the daf-2 insulin/IGF-1 signaling pathwayCitation18 (see Ewbank 2006 and Irazoqui and Ausubel 2010,Citation54,Citation55 for more extensive reviews of these and other C. elegans immune signaling pathways). The p38 MAPK pathway () acts autonomously in the epidermis and intestine to regulate antimicrobial gene expression.Citation56,Citation57 In contrast, the dbl-1/TGFβ and daf-2/insulin pathways act in the epidermis and intestine, respectively, but are regulated by non-autonomous signals from neurons ( and C).Citation53,Citation58 For example, the daf-2 receptor is expressed and functions in the intestine; it is regulated by secretion of the ins-7 insulin-like ligand from neurons.Citation58 The p38 MAPK and daf-2 pathways act in parallel to control overlapping but different sets of genes to fight infection.Citation44,Citation50 In general, daf-2 regulates general stress response genes, while the effect of p38 MAPK is more specific to infection.Citation50 In addition to controlling the nematode innate immune response, these pathways are themselves potential targets for pathogens to inhibit the immune response, as P. aeruginosa can activate daf-2 signaling, thereby inhibiting production of some antimicrobials.Citation59
daf-2 mutant nematodes live long and are resistant to numerous environmental stresses. All these effects are dependent on the daf-16 transcription factor, as evidenced by experiments using daf-2; daf-16 double mutant nematodes. This relationship extends to the regulation of pathogen resistance as well (), with the daf-16 mutation suppressing the enhanced pathogen resistance of the daf-2 mutant.Citation18 However, mutations in certain other kinases that act downstream of daf-2 are able to separate the effects of insulin signaling on lifespan and innate immunity. While nematodes harboring mutations in either pdk-1 or sgk-1 are long-lived, they are not resistant to pathogen.Citation22 In contrast, mutation of either akt-1 or akt-2 has a minimal effect on C. elegans lifespan but does induce strong pathogen resistance.Citation22 This ability to separate the effects of insulin signaling on lifespan and innate immunity suggests that other stresses besides pathogen infection are critical to overall lifespan regulation, at least in this context.
Germline proliferation also controls both lifespan and innate immunity in C. elegans, as sterile nematodes are resistant to both Gram-negative and Gram-positive bacteria.Citation21–Citation23 The long lifespan of sterile nematodes is completely dependent on daf-16.Citation19,Citation20 However, the effect of daf-16 in relation to germline proliferation and innate immunity varies depending on the growth conditions of the pathogen. Under certain conditions, the pathogen resistance of sterile nematodes is completely dependent on daf-16 ()Citation22,Citation23 while under other conditions, it is completely independent.Citation21 Again, there is no absolute correlation between long life and pathogen resistance. The germline also acts in part or in whole in parallel to the p38 MAPK pathway to control pathogen resistance ().Citation21 While there is a general correlation between lifespan and pathogen resistance in C. elegans and related species,Citation60,Citation61 the complicated genetic interactions suggest that there is no simple causal interaction between the two.
Evolutionary Conservation
At first glance, it might be tempting to dismiss the genetic experiments in C. elegans as having no real bearing on human aging. However, experiments in other organisms, including Drosophila, mice and even humans, suggest the pathways that affect C. elegans lifespan also affect lifespan and stress resistance in other organisms, including mammals and thus they theoretically offer the potential as therapeutic targets to delay aging and aging-related diseases.
As in C. elegans, the insulin/IGF-1 pathway controls lifespan, stress resistance and pathogen resistance in Drosophila. Mutation of the insulin receptor in flies increases lifespan.Citation62 Flies carrying mutations in the insulin-like ligand chico are also long-lived, resistant to some but not all stresses (such as starvation but not thermal stress)Citation63 and are resistant to the bacterial pathogens P. aeruginosa and E. faecalis.Citation64 There is also evidence for the role of the insulin and IGF-1 signaling pathways in lifespan regulation in mammals. While completely knocking out the insulin-signaling pathway has profoundly detrimental consequences, partially turning down the pathway using heterozygous animals or knockouts in particular tissues has allowed investigators to test the function of these pathways in lifespan regulation in mice. These studies have demonstrated that several components in these pathways, including the insulin receptor, the Igf1 receptor and IRS1 (Insulin receptor substrate 1), all affect lifespan;Citation65–Citation68 mutation of some of these signaling components also increases resistance to oxidative stress in mice.Citation66,Citation69 There is even evidence suggesting a role of insulin signaling in lifespan regulation in humans; studies of long-lived individuals have led to the identification of DNA polymorphisms in insulin/IGF-1 signaling components that are associated with longevity.Citation70–Citation75 Thus, there is strong evidence in multiple species that the role of the insulin/IGF-1 pathway in lifespan regulation and stress resistance is evolutionarily conserved.
There is also some evidence suggesting that the germline can regulate lifespan in organisms besides C. elegans, although this evidence is so far not as compelling as the evidence for the insulin/IGF-1 pathway. The germline, at least under some conditions, can modulate lifespan in Drosophila.Citation76 Similarly, the germline may affect lifespan and stress resistance in mammals. Transplantation of young ovarian tissue into older mice leads to an increase in mouse lifespan,Citation77 and postponement of menopause in mice can delay some age-related health complications.Citation78 Obviously there is limited evidence that the germline can affect lifespan in humans. The extension of lifespan in nematodes without a germline is dependent on the somatic gonad,Citation20 indicating that the somatic gonad plays a protective role in lifespan regulation in C. elegans. Interestingly, while removal of ovaries in post-menopausal women has a positive survival effect on those at risk of ovarian and breast cancer, this surgery has an overall net negative effect on long-term health, with increased risk of most other diseases, such as cardiovascular disease and other cancers.Citation79–Citation81 This raises the possibility that somatic gonadal tissue in humans may also serve a protective function.Citation5
The Central Role of FOXO-Family Transcription Factors
The DAF-16/FOXO family of transcription factors plays a key role in regulating lifespan, stress resistance and immunity in many systems. As outlined above, daf-16 plays a critical role in all these processes in C. elegans, acting downstream of multiple signaling pathways. The role of daf-16 includes the regulation of antimicrobial gene expression during infection.Citation39,Citation40 Likewise, the Drosophila FOXO transcription factor regulates antimicrobial gene expression.Citation82 In mammals, different forkhead family transcription factors control different aspects of immune cell development and function, including the development of regulatory T cells, the regulation of T cell tolerance and thymic development.Citation83,Citation84 FOXO also plays a role in the aging of the immune system in mammals. Dis-regulation of the NFκB transcription factor is thought to play a critical role in inflamm-aging,Citation85,Citation86 and FoxO3a in turn has been shown to inhibit NFκB activity.Citation87–Citation89 Thus, the DAF-16/FOXO transcription factor is at the center of key lifespan and stress response pathways in multiple organisms, and the study of its regulation in the simpler model systems should provide useful insights into the role of forkhead family transcription factors in mammals.
The Relationship Between Stress, Immunity and Aging
What causes aging has been a profound question that has sparked much debate and numerous theories. One current widely-accepted theory is that oxidative damage caused by free radicals causes aging.Citation90,Citation91 The studies using long-lived mutants in model organisms have largely backed up these ideas, as most long-lived mutants are resistant to one or multiple stresses. However, there are some exceptions, and mutant combinations or treatments have been identified that uncouple lifespan regulation from resistance to one or multiple stresses, arguing against a simple model in which any single stress is the key factor in aging. This is illustrated by experiments dealing with the role of oxidative damage in aging. For example, the mev-4 mutation renders C. elegans long-lived and resistant to oxidative damage; however the mev-4; daf-16 double mutant is no longer long-lived but is still resistant to oxidative damage.Citation92 If oxidative damage were the sole factor in lifespan regulation, then the double mutant would be expected to live long. An even more striking effect is observed with the germline. Nematodes lacking a germline that also lack daf-16 activity no longer live long but are still resistant to oxidative stress.Citation93 What is interesting about sterilized daf-16 mutant nematodes is that they are also still resistant to thermal stress and, at least under some assay conditions, pathogen infection.Citation21,Citation94 So sterilized daf-16 mutant nematodes are resistant to oxidative stress, thermal stress and pathogen infection; yet these animals still do not live long! There may be an additional critical stress that affects longevity in these animals, but no single stress is an obvious candidate. Further muddying the role of oxidative stress in lifespan regulation are experiments in C. elegans and mice in which the SOD genes are either mutated or overexpressed.Citation95–Citation97 While there are some caveats to these experiments, the correlation between lifespan and SOD activity in nematodes and mice is contradicted if not completely disproven in these experiments. It is formally possible that oxidative stress could be a key determinant of aging in wild-type animals or in some mutants but not in others. These possibilities will keep investigators busy researching and debating for quite some time. What is clear is that stress, immunity and aging are inter-related; altering one process often perturbs the others, and some of the same signaling pathways and components are shared. A simple global theory that explains how and why is lacking. Undoubtedly, experimentation in model organisms will continue to reap rewards in addressing these issues.
Figures and Tables
Figure 1 Signaling pathways that regulate longevity in C. elegans. The daf-2 insulin/IGF-1-like signaling pathway (A) and germline proliferation (B) both regulate the DAF-16/FOXO family transcription factor, which in turn regulates nematode longevity.
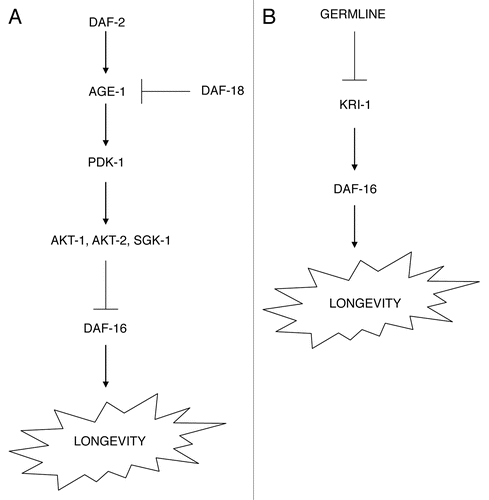
Figure 2 Innate immunity regulation in C. elegans. Three key signaling pathways regulate innate immunity in C. elegans. The p38 MAPK cascade functions in the intestine and epidermis to regulate antimicrobial gene expression, and includes TIR-1, a protein with a conserved TIR domain, the MAPKKK NSY-1, the MAPKK SEK-1 and the MAPK PMK-1 (A). How this pathway senses pathogens is still unclear, but the specificity of the nematode innate immune response suggests that several pathogen recognition receptors (PRRs) exist in the genome. The TGFβ family member DBL-1 is synthesized in neurons and controls expression of antimicrobial genes in the epidermis (B). DBL-1 signals via the TGFβ receptor (composed of DAF-4 and SMA-6) and a downstream SMAD (SMA-3). Neuronal expression of the insulin-like peptide INS-7 also regulates antimicrobial gene expression non-autonomously, acting on the DAF-2 receptor and downstream signaling components, including DAF-16 in the intestine (C).
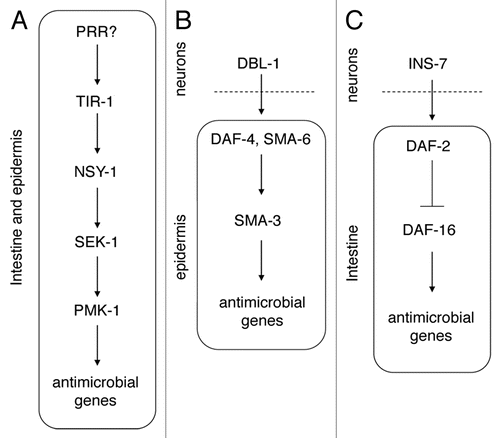
Figure 3 Model for the regulation of lifespan and innate immunity. The daf-2 (A) and germline (B) pathways both regulate lifespan and innate immunity in C. elegans. The effect of daf-2 on both lifespan and innate immunity depends on the DAF-16 transcription factor (A). Similarly, the effect of the germline on lifespan also requires daf-16. However, depending on the growth conditions of the pathogen and the nematodes, the effect of the germline on innate immunity may or may not depend on DAF-16 (B). The germline also acts in parallel to a p38 MAPK pathway to regulate innate immunity. This figure was originally published in Alper et al. “The Caenorhabditis elegans germ line regulates distinct signaling pathways to control lifespan and innate immunity.” J Biol Chem 2010; 285:1822–8.
Acknowledgements
I thank Danielle Garsin, Queelim Ch'ng and Jennifer Kemp for critical reading of this manuscript.
References
- Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol 2008; 43:718 - 728
- Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int 2009; 22:1041 - 1050
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000; 908:244 - 254
- Wood WB. The Nematode Caenorhabditis elegans 1988; New York Cold Spring Harbor Laboratory Press
- Kenyon CJ. The genetics of ageing. Nature 2010; 464:504 - 512
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 1988; 118:75 - 86
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature 1993; 366:461 - 464
- Klass MR. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev 1983; 22:279 - 286
- Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. Faseb J 2001; 15:627 - 634
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, et al. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 1998; 150:129 - 155
- Giannakou MEPL. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci 2007; 32:180 - 188
- Honda Y, Honda S. Oxidative stress and life span determination in the nematode Caenorhabditis elegans. Ann N Y Acad Sci 2002; 959:466 - 474
- Hyun M, Lee J, Lee K, May A, Bohr VA, Ahn B. Longevity and resistance to stress correlate with DNA repair capacity in Caenorhabditis elegans. Nucleic Acids Res 2008; 36:1380 - 1389
- Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci USA 1993; 90:8905 - 8909
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA 1995; 92:7540 - 7544
- Murakami S, Johnson TE. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics 1996; 143:1207 - 1218
- Vanfleteren JR. Oxidative stress and ageing in Caenorhabditis elegans. Biochem J 1993; 292:605 - 608
- Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, et al. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 2003; 300:1921
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science 2002; 295:502 - 505
- Hsin HKC. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 1999; 399:362 - 366
- Alper S, McElwee M, Apfeld J, Lackford B, Freedman J, Schwartz D. The Caenorhabditis elegans germ line regulates distinct signaling pathways to control lifespan and innate immunity. J Biol Chem 2010; 285:1822 - 1828
- Evans E, Chen W, Tan M. The DAF-2 Insulin-like Signaling Pathway Independently Regulates Aging and Innate Immunity in C. elegans. Aging Cell 2008; 7:879 - 893
- Miyata S, Begun J, Troemel ER, Ausubel FM. DAF-16-dependent suppression of immunity during reproduction in Caenorhabditis elegans. Genetics 2008; 178:903 - 918
- Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol 2006; 190:191 - 202
- Panowski SH, Dillin A. Signals of youth: endocrine regulation of aging in Caenorhabditis elegans. Trends Endocrinol Metab 2009; 20:259 - 264
- Russell SJKC. Endocrine regulation of ageing. Nat Rev Mol Cell Biol 2007; 8:681 - 691
- Wolff S, Dillin A. The trifecta of aging in Caenorhabditis elegans. Exp Gerontol 2006; 41:894 - 903
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997; 277:942 - 946
- Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev 2003; 17:844 - 858
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev 2001; 15:672 - 686
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 1996; 382:536 - 539
- Hertweck M, Gobel C, Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell 2004; 6:577 - 588
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev 1999; 13:1438 - 1452
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev 1998; 12:2488 - 2498
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol 2001; 11:1950 - 1957
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 1997; 278:1319 - 1322
- Lin KHH, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet 2001; 28:139 - 145
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997; 389:994 - 999
- McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell 2003; 2:111 - 121
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003; 424:277 - 283
- Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell 1998; 2:887 - 893
- Berman JRKC. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 2006; 124:1055 - 1068
- Fares H, Greenwald I. Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics 2001; 159:133 - 145
- Alper S, McBride SJ, Lackford B, Freedman JH, Schwartz DA. Specificity and complexity of the C. elegans innate immune response. Mol Cell Biol 2007; 27:5544 - 5553
- Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, et al. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol 2004; 5:488 - 494
- Liberati NT, Fitzgerald KA, Kim DH, Feinbaum R, Golenbock DT, Ausubel FM. Requirement for a conserved Toll/interleukin-1 resistance domain protein in the Caenorhabditis elegans immune response. Proc Natl Acad Sci USA 2004; 101:6593 - 6598
- Mallo GV, Kurz CL, Couillault C, Pujol N, Granjeaud S, Kohara Y, et al. Inducible antibacterial defense system in C. elegans. Curr Biol 2002; 12:1209 - 1214
- O'Rourke D, Baban D, Demidova M, Mott R, Hodgkin J. Genomic clusters, putative pathogen recognition molecules and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res 2006; 16:1005 - 1016
- Shapira M, Hamlin BJ, Rong J, Chen K, Ronen M, Tan MW. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci USA 2006; 103:14086 - 14091
- Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet 2006; 2:183
- Darby C. Interactions with microbial pathogens. Wormbook 2005; http://www.wormbook.org
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 2002; 297:623 - 626
- Zugasti O, Ewbank JJ. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGFbeta signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol 2009; 10:249 - 256
- Ewbank JJ. Signaling in the immune response. Wormbook 2006; http://www.wormbook.org
- Irazoqui J, Ausubel F. 99th Dahlem Conference on infection, inflammation and chronic inflammatory disorders: Caenorhabditis elegans as a model to study tissues involved in host immunity and microbial pathogenesis. Clinical and Experimental Immunology 2010; 160:48 - 57
- Bolz DD, Tenor JL, Aballay A. A conserved PMK-1/p38 MAPK is required in C. elegans tissue-specific immune response to Y. pestis infection. J Biol Chem 2010; 285:10832 - 10840
- Pujol N, Link EM, Liu LX, Kurz CL, Alloing G, Tan MW, et al. A reverse genetic analysis of components of the Toll signaling pathway in Caenorhabditis elegans. Curr Biol 2001; 11:809 - 821
- Kawli T, Tan MW. Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol 2008; 9:1415 - 1424
- Evans EA, Kawli T, Tan MW. Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog 2008; 4:1000175
- Amrit FR, Boehnisch CM, May RC. Phenotypic covariance of longevity, immunity and stress resistance in the Caenorhabditis nematodes. PLoS One 5:9978
- van den Berg MC, Woerlee JZ, Ma H, May RC. Sex-dependent resistance to the pathogenic fungus Cryptococcus neoformans. Genetics 2006; 173:677 - 683
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001; 292:107 - 110
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 2001; 292:104 - 106
- Libert S, Chao Y, Zwiener J, Pletcher SD. Realized immune response is enhanced in long-lived puc and chico mutants but is unaffected by dietary restriction. Mol Immunol 2008; 45:810 - 817
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 2003; 299:572 - 574
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 2003; 421:182 - 187
- Kappeler L, De Magalhaes Filho C, Dupont J, Leneuve P, Cervera P, Perin L, et al. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol 2008; 6:254
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J 2008; 22:807 - 818
- Baba T, Shimizu T, Suzuki Y, Ogawara M, Isono K, Koseki H, et al. Estrogen, insulin and dietary signals cooperatively regulate longevity signals to enhance resistance to oxidative stress in mice. J Biol Chem 2005; 280:16417 - 16426
- Bonafe M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C, et al. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. Journal of Clinical Endocrinology and Metabolism 2003; 88:3299 - 3304
- Franceschi C, Olivieri F, Marchegiani F, Cardelli M, Cavallone L, Capri M, et al. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech Ageing Dev 2005; 126:351 - 361
- Kojima T, Kamei H, Aizu T, Arai Y, Takayama M, Nakazawa S, et al. Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways. Experimental Gerontology 2004; 39:1595 - 1598
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proceedings of the National Academy of Sciences of the United States of America 2008; 105:3438 - 3442
- van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, et al. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell 2005; 4:79 - 85
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, et al. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA 2008; 105:13987 - 13992
- Flatt T, Min KJ, D'Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, et al. Drosophila germ-line modulation of insulin signaling and lifespan. Proc Natl Acad Sci USA 2008; 105:6368 - 6373
- Cargill SLCJ, Müller HG, Anderson G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell 2003; 2:185 - 190
- Perez GIJA, Wise L, Lipina T, Kanisek M, Bechard A, Takai Y, et al. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci USA 2007; 104:5229 - 5234
- Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet Gynecol 2009; 113:1027 - 1037
- Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Clin Obstet Gynecol 2007; 50:354 - 361
- Rivera CM, Grossardt BR, Rhodes DJ, Brown RD Jr, Roger VL, Melton LJ 3rd, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009; 16:15 - 23
- Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, et al. FOXO-dependent regulation of innate immune homeostasis. Nature 463:369 - 373
- Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol 2004; 4:889 - 899
- Jonsson H, Peng SL. Forkhead transcription factors in immunology. Cell Mol Life Sci 2005; 62:397 - 409
- Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NFκB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev 2008; 7:83 - 105
- Spencer NF, Poynter ME, Im SY, Daynes RA. Constitutive activation of NFkappaB in an animal model of aging. Int Immunol 1997; 9:1581 - 1588
- Lin L, Hron JD, Peng SL. Regulation of NFkappaB, Th activation and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 2004; 21:203 - 213
- Peng SL. Immune regulation by Foxo transcription factors. Autoimmunity 2007; 40:462 - 469
- Salminen A, Ojala J, Huuskonen J, Kauppinen A, Suuronen T, Kaarniranta K. Interaction of aging-associated signaling cascades: inhibition of NFkappaB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci 2008; 65:1049 - 1058
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev 1998; 78:547 - 581
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956; 11:298 - 300
- Fujii M, Matsumoto Y, Tanaka N, Miki K, Suzuki T, Ishii N, et al. Mutations in chemosensory cilia cause resistance to paraquat in nematode Caenorhabditis elegans. J Biol Chem 2004; 279:20277 - 20282
- Yamawaki TM, Arantes-Oliveira N, Berman JR, Zhang P, Kenyon C. Distinct Activities of the Germline and Somatic Reproductive Tissues in the Regulation of Caenorhabditis elegans Longevity. Genetics 2008; 178:513 - 526
- Libina NBJ, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 2003; 115:489 - 502
- Doonan R, McElwee JJ, Matthijssens F, Walker GA, Houthoofd K, Back P, et al. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev 2008; 22:3236 - 3241
- Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, et al. Is the oxidative stress theory of aging dead?. Biochim Biophys Acta 2009; 1790:1005 - 1014
- Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet 2009; 5:1000361