Abstract
Dendrites and spines are key regulators of neuronal function often affected in cognitive disorders. Neuronal subclasses are characterized by a wide range of dendritic morphologies that aid their specific functions. However, how subclass-specific dendritic trees arise during vertebrate development remains largely unknown. We have recently reported that the restricted expression of Cux1 and Cux2 genes in the upper layers of the cerebral cortex determines the specific morphology of dendrites and spines and the function of these neurons. Since Cux genes are the vertebrate homologues of Drosophila Cut, which specifies the dendritic morphologies of certain sensory neuron populations, our findings suggest that mechanisms of dendrite differentiation are conserved between Drosophila and mammals, which had yet to be demonstrated. Importantly, we found that Cux genes not only modulate dendritic branching, but also dendritic spine morphogenesis, the functional synapse, and cognition. Dendritic spine stabilization was partly mediated by direct repression of genes of the Xlr family, previously implicated in cognitive defects in a model of Turner syndrome. Hence, our work indicates that neuronal subclass specific determinants may intrinsically affect synaptic activity beyond expected. The functions of Cux1 and Cux2 were additive, and complement each other to establish the final pattern of the dendritic tree and the number and strength of the synapses. This work unravels novel mechanisms of dendritogenesis and synaptogenesis and illustrates how regulating dendritic structures contributes to the specialization of upper layer neurons. It will be interesting to dissect how these mechanisms regulate cortical activity, area specialization and cognitive functions.
Dendritic branches and spines are key regulators of neuronal function. Number, growth and orientation of dendritic branches modify the way information is integrated and select axonal targets.Citation1,Citation2 Dendritic spines are specialized structures that modulate the activity, strength and stability of the synapse.Citation3–Citation5 Both structures are often altered in number and morphology in mental disorders.Citation6–Citation8 From the times of Cajal we learned to identify the myriad of different types of neurons by their stereotyped dendritic morphologies, and we now begin to understand how these dendritic patterns serve their specialized functions.Citation1 However, how subclass-specific neuronal features are defined during development is largely unknown. Based in studies on Drosophila, dendritic architecture is thought to be instructed by the selective expression of transcription factors (TFs), but few of such TFs have been described in vertebrates.Citation2,Citation9 Moreover, the possibility that these subclass specific intrinsic factors may affect the formation of dendritic spines and synapses has not been proposed or explored.
Cux1 and Cux2 are two transcription factors selectively expressed in the pyramidal neurons of the upper layers (II, III and IV) of the mouse cortex.Citation10,Citation11 These neurons have elaborated dendritic morphologies and profusion of spinesCitation12 that allow them to integrate intracortical circuits involved in the higher cognitive tasks of the brain. They might be considered as a late evolutive addition, since they appear in mammals and are highly represented in the primate cerebral cortex, especially in humans.Citation13 We have recently found that Cux genes exert an intrinsic control of the dendritic structures of the upper layer neurons of the mouse cerebral cortex ().Citation15 Our knockout and knockdown studies demonstrate that the homeobox Cux1 and Cux2 are early regulators of dendrite branching in a cell autonomous manner. Cux genes are the vertebrate homologs of Drosophila homeobox Cut, which specifies the dendritic morphology of certain sensory neurons.Citation14 Our findings support the existence of conserved mechanisms of dendritic differentiation between flies and mammals. They may also imply that the activity of Drosophila Cut in specifying simpler neuronal types might have been co-opted during cortical evolution to generate the more complex neurons of mammals. But perhaps more unexpected, we found that Cux1 and Cux2 instruct also genetic programs that control the number and morphology of the dendritic spines. In the absence of Cux genes, the dendritic spines adopt a more immature morphology, with longer necks and smaller heads. Correspondingly, electrophysiological recordings show reduced number and strength of the synapses. A few other TFs, such as MEF2, have been previously implicated in activity dependent spine formation and synaptogenesis,Citation8,Citation16,Citation17 but these mechanisms apply to most neuronal populations. The implication of Cux1 and Cux2 on neuronal plasticity and in normal brain function remains to be understood. Why is it important to selectively restrict or promote synapse formation by intrinsic factors? Do presynaptic axons need this constrains to define their connectivity?
Another interesting point is the additive and complementary functions of Cux1 and Cux2. Cux1 and Cux2 label most neurons of the superficial layers and display overlapping patterns of expression in several areas of the cortex. This indicates that they are likely co-expressed in many upper layer neurons, as we formally corroborated for the neurons of the somatosensory cortex. Initially, this suggested us that Cux1 and Cux2 might have redundant functions.Citation10,Citation18 However, we found that Cux1 and Cux2 are complementary but not redundant. Upper layer neurons of the somatosensory cortex of both Cux1 and Cux2 single mutants show similar reduction in dendritic complexity and comparable defects in dendritic spine numbers and morphologies. Double loss of Cux1 and Cux2 expression induced more dramatic defects in dendrites and spines. Ectopic expression of Cux1 in cingulated neurons, that normally express Cux2 but lower levels of Cux1, increased branching and reproduced the more complex dendritic morphologies of the somatosesory areas. Therefore, we concluded that the functions of Cux genes add to each other to stimulate branching and that it is the combinatorial expression of Cux1 and Cux2 that defines the final dendritic pattern.
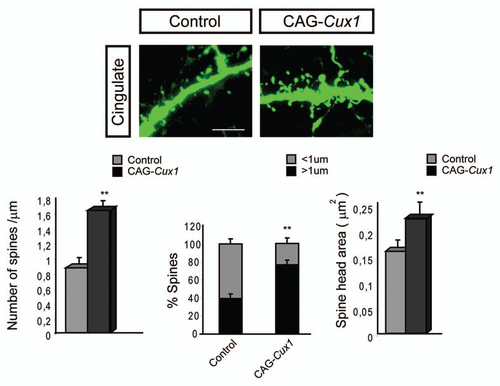
Out of these experiments, it should be highlighted that overexpression of Cux1 also incremented dendritic spine density and induced more mature dendritic spine phenotypes (). Thus, the additive functions were revealed also for the intrinsic control of the spine. Altogether, these suggest that discrete differences in the levels of expression of Cux1 and Cux2 may modulate dendritic and spine morphogenesis in a dose-dependent manner in subsets of superficial neurons or regionally, in cortical areas. This would refine their functions and establish a fine tuning of their connectivity.
In search for the downstream elements by which Cux genes exert their functions we found mechanisms of synaptogenesis key to cognition, including the regulation of NMDAR2B and PSD95.Citation8,Citation19–Citation21 Directly downstream of Cux, we found the chromatin remodeling genes of the Xlr family (). These genes were initially identified as upregulated in the Cux2-/- cortex in a screen of genes differentially expressed. Previous report showed that increased level of Xlr3b and Xlr4b expression correlated with more acute behavioral inflexibility in a mouse model of Turner syndrome.Citation22,Citation23 Nothing was known about the functions of Xlr genes, but dendrite and spine defects associate to mental retardation and therefore, it seemed possible that these genes were involved in the changes in dendritic structures in the absence of Cux. Further research identified Cux binding sites in the Xlr3b and Xlr4b locus and proved that Cux proteins bind to these sites in vivo and repress Xlr expression. The functional demonstration of the direct implication of Xlr genes in the control of the synapse was provided by experiments in which RNAs of interference targeting Xlr rescued normal spine density and reduced the proportion of long spines upon Cux loss of function. Dendritic tree was not affected, proving to be independently regulated. Interestingly, Cux1 and Cux2 proteins selectively bound and repressed distinct regulatory regions on Xlr3b and Xlr4b loci, illustrating the mechanisms conveying the additive functions (, lower parts).
The expression of Cux2 selectively defines the upper layer of the human cortex.Citation24 We identified FAM9A, B and CCitation25 as the closest orthologos of Xlr genes in human and found sequences containing Cux binding sites in FAM9A, B and C loci that are conserved between primates and humans. In vitro ChIP experiments in human cell lines demonstrated binding of Cux1 and Cux2 proteins to these regions, indicating that it is possible that similar Cux mediated synaptic mechanisms act in humans.
The functions of Cux in spine morphogenesis highlight the existence of neuronal subclass specific mechanisms of synaptogenesis that contribute to the establishment of cognitive circuits. Accordingly, we found defects in working memory in Cux2-/-. Work lies ahead to further investigate these intrinsic mechanisms of synapse regulation. It will likely reveal genes and proteins affected in cognitive disorders and neurodegeneration. In addition, it will be a challenge to investigate how the selective control of dendritic structures and spines by Cux1 and Cux2 contribute to laminar, columnar and area connectivity, and ultimately to the establishment of the intellectual capabilities that rest in the cortex.
Figures and Tables
Figure 1 Cux genes control dendrite branching and synaptogenesis. Cux1 and Cux2 regulate neuronal differentiation and control intrinsic mechanisms of dendrite development, spine formation and synaptic function in upper layers in the cortex. Upper part: Dendritic parterns in WT and Cux2-/- pyramidal neurons of the upper layers. Lower parts: Downregulation of Xlr3b and Xlr4b gene expression by Cux proteins contributes to dendritic spine differentiation. Cux1 and Cux2 bind and regulate different regions in the Xlr4b locus. Miniature excitatory postsynaptic current (mEPSCs) from layer II and III pyramidal cells of Cux2-/- mice were reduced in amplitude and frequency.
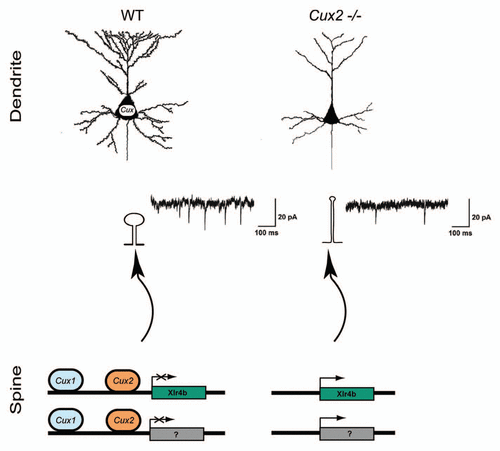
Figure 2 Dendritic spine formation in neurons of the cingulate cortex is stimulated upon Cux1 overexpression. Upper parts show representative confocal image of GFP positive spines in the cingulate cortex. These neurons had been electroporated with control or CAG-Cux1 plasmid. Scale bar represents 5 um. Lower parts show quantification of dendritic spine number, spine morphology and spine head area. Data in bar graphs depict mean ± SD. *p < 0.005, **p < 0.001, compared with control. This figure is a modification of Cubelos et al.Citation15
Acknowledgements
This work was supported by the MICINN grants SAF2008-00211; PIE-200820I166, and a grant from the Spanish Comunidad de Madrid CCG08-CSIC/SAL-3464. B. Cubelos holds a fellowship from the CSIC (JAEDoc2008-020).
References
- van Elburg RA, van Ooyen A. Impact of dendritic size and dendritic topology on burst firing in pyramidal cells. PLoS Comp Biol 2010; 6:1000781
- Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance and remodeling of dendritic fields. Ann Rev Neurosci 2007; 30:399 - 423
- Majewska A, Brown E, Ross J, Yuste R. Mechanisms of calcium decay kinetics in hippocampal spines: role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J Neurosci 2000; 20:1722 - 1734
- Noguchi J, Matsuzaki M, Ellis-Davies GC, Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron 2005; 46:609 - 622
- Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci 2004; 5:24 - 34
- Dierssen M, Ramakers GJ. Dendritic pathology in mental retardation: from molecular genetics to neurobiology. Genes, Brain, Behav 2006; 2:48 - 60
- Boda B, Dubos A, Muller D. Signaling mechanisms regulating synapse formation and function in mental retardation. Curr Opin Neurobiol 2010; 20:519 - 527
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol 2006; 16:95 - 101
- Chen JG, Rasin MR, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci USA 2005; 102:17792 - 17797
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J Comp Neurol 2004; 479:168 - 180
- Zimmer C, Tiveron MC, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex 2004; 14:1408 - 1420
- DeFelipe J, Jones EG. Cajal on the Cerebral Cortex 1988; New York Oxford University Press
- Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature 2005; 437:64 - 67
- Grueber WB, Jan LY, Jan YN. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell 2003; 112:805 - 818
- Cubelos B, Sebastian-Serrano A, Beccari L, Calcagnotto ME, Cisneros E, Kim S, et al. Cux1 and Cux2 regulate dendritic branching, spine morphology and synapses of the upper layer neurons of the cortex. Neuron 2010; 66:523 - 535
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 2006; 311:1008 - 1012
- Shalizi A, Gaudilliere B, Yuan Z, Stegmuller J, Shirogane T, Ge Q, et al. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 2006; 311:1012 - 1017
- Cubelos B, Sebastian-Serrano A, Kim S, Moreno-Ortiz C, Redondo JM, Walsh CA, et al. Cux-2 controls the proliferation of neuronal intermediate precursors of the cortical subventricular zone. Cereb Cortex 2008; 18:1758 - 1770
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science 2000; 290:1364 - 1368
- Ultanir SK, Kim JE, Hall BJ, Deerinck T, Ellisman M, Ghosh A. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci USA 2007; 104:19553 - 19558
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 2010; 65:373 - 384
- Raefski AS, O'Neill MJ. Identification of a cluster of X-linked imprinted genes in mice. Nat Genet 2005; 37:620 - 624
- Davies W, Isles A, Smith R, Karunadasa D, Burrmann D, Humby T, et al. Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects on cognitive function in mice. Nat Genet 2005; 37:625 - 629
- Arion D, Unger T, Lewis DA, Mirnics K. Molecular markers distinguishing supragranular and infragranular layers in the human prefrontal cortex. Eur J Neurosci 2007; 25:1843 - 1854
- Martinez-Garay I, Jablonka S, Sutajova M, Steuernagel P, Gal A, Kutsche K. A new gene family (FAM9) of low-copy repeats in Xp22.3 expressed exclusively in testis: implications for recombinations in this region. Genomics 2002; 80:259 - 267