Abstract
In neurons and neurosecretory (nerve) cells, neurite outgrowth requires the enlargement of the plasma membrane sustained by the exocytosis of specific vesicles. The well known, slow canonical form of outgrowth induced in pheochromocytoma PC12 cells by NGF, as well as the outgrowth taking place in neurons, involve vesicles positive for the vSNARE Ti-VAMP. Working in defective PC12 clones expressing high levels of the transcriptional repressor REST, we have identified now a new, rapid form of outgrowth, triggered by activation of a small GTPase, Rac1. This form is sustained by the exocytosis of another type of vesicles, taking place locally at the tip of neurite growth cones, the enlargeosomes (vSNARE: VAMP4). This new form, which is positively controlled by REST, requires the dynamics of microtubules, but not of microfilaments. Its signalling remains undefined because established second messengers, (Ca2+, DAG, cAMP) seem not involved. Using a high REST/enlargeosome-rich PC12 clone transfected with TrkA we have found that the NGF-induced outgrowth is not always slow, but can be fast in cells expressing high levels of the receptor involved, TrkA; that PC12 can express together the two distinct forms of outgrowth, canonical and new, activated independently from each other. Their comparative characterization in terms of changes in the cytoskeleton has now been initiated. The two forms are present also in neurons where the new one seems to predominate in the initial phases of development, the canonical one later on. Our results identify a new aspect of the REST impact in nerve cell specificity/function. The existence of two distinct forms of neurite outgrowth may cope better than a single form with the variable needs of nerve cells in the subsequent stages of their development.
The outgrowth of neurites and their subsequent differentiation into axons and dendrites is one of the most important properties of neurons. The process has been revealed in detail by studies carried out by Gary Banker and his associates in primary hippocampal neuronal cultures. Five subsequent steps were identified, corresponding first to the sprouting of multiple neurites similar to each other in structure and composition, then to their outgrowth, to the differentiation of one such neurites into the axon, to the differentiation of the others into dendrites and, finally, to the establishment of synapses and networks.Citation1–Citation4 Neurosecretory cells, such as those of the adrenal medulla and of various nerve tumors, can also sprout neurites in processes known as neuron-like differentiation.Citation5,Citation6 In these cells, however, differentiation stops at step 2 and all neurites remain similar to each other.
In order outgrowth to start, at least two processes need to take place: the reorganization of the cytoskeleton, with longitudinal distribution of microtubules and concentration of actin filaments in the phylopodia and lamellipodia of growth cones,Citation7,Citation8 and the enlargement of the cell surface sustained by the exocytosis of cytoplasmic vesicles.Citation7,Citation9–Citation12 The nature of these vesicles, named plasmalemma precursor vesicles,Citation7 has remained largely undefined except for their distinction from the vesicles containing neurotransmitters. The only marker identified so far is Ti-VAMP, a vSNARE insensitive to the chlostridial toxins participating in the exocytic complex of these vesicles.Citation7,Citation9–Citation11 Unfortunately Ti-VAMP is not specific for the plasmalemma precursor vesicles, but is present in other organelles such as endosomes and lysosomes. We will define the outgrowth sustained by the Ti-VAMP-positive vesicles as the canonical form of outgrowth. Whether other forms also exist was unknown.
In order to investigate the outgrowth vesicles we have worked primarily in two groups of PC12 clones, wild-type (wt) and defective of neurosecretion. PC12, a line developed over 30 years ago from a rat pheochromocytoma, is the most popular model of neurosecretory cell.Citation5,Citation6 The main differences between wt and defective clones depend on the differential expression of the transcription factor REST/NRSF. The latter is the master factor that orchestrates differentiation of neurons. It is high in stem cells and in most nonnerve cells; it decreases rapidly to low levels in the course of neuron and nerve cell differentiation. Low REST governs the expression of numerous specific genes and therefore the appearance of the typical, nerve cell phenotype.Citation13 Defective PC12 are neurosecretory cells that express high levels of REST.Citation14 In addition to the defect in neurosecretion they lack other components typical of nerve cells, such as various channels and receptors, and show no neurite sprouting in response to the classical stimulatory agent, NGF.Citation15
Previous studies had suggested the latter defect to depend on the lack of the NGF receptor, TrkA. The subsequent signaling cascade could in contrast be active.Citation15 In order to investigate the latter possibility we decided to bypass the receptor, stimulating the outgrowth via specific drug activation of a small GTPase, Rac1, known to participate in the signaling cascade. The results were astonishing. In the defective PC12, neurite outgrowth was seen to start a few min after Rac1 activation, yielding long fibers within 1–2 hrs, i.e., much faster than the outgrowth induced by NGF in wt PC12, which requires 1–2 days. The fast, Rac1-dependent response was due to the “explosive” exocytosis of small vesicles, the enlargeosomes, that we had discovered in these cells years ago and that exist in numerous types of non-nerve cells.Citation16 The Rac1-dependent outgrowth was found to occur even when the defective PC12 were exposed to a variety of inhibitions. Increases of second messengers, such a Ca2+, cAMP and diacylglycerol, were not needed.
The outgrowth responses induced by NGF and by Rac1 activation were investigated also in a defective PC12 clone transfected with TrkA. In these cells not only the outgrowth response induced by Rac1, but also that induced by NGF occur within tenth of min. This allowed us to investigate the two types of responses in parallel and in a single cell type. The results were quite clear. NGF was shown to induce the exocytosis of vesicles positive for Ti-VAMP, i.e., those that are believed to sustain the canonical form of outgrowth; Rac1 the exocytosis of enlargeosomes, which express another type of VAMP, also insensitive to chlostridial toxins, VAMP4.Citation17 Therefore, the defective PC12 cells transfected with TrkA possess two types of vesicles competent to sustain neurite outgrowth, positive for distinct types of VAMP, active in distinct exocytic and outgrowth processes.
In order to grow, neurites require not only the expansion of their plasma membrane but also the restructuring of their cytoskeleton. The duality of the membrane traffic processes sustaining outgrowth might therefore be accompanied by a duality of the cytoskeletal reorganization. The simple use of drugs such as nocodazole, latrunculinA and jusplakinolide, has revealed that dynamic microtubules are needed for outgrowth to occur, whereas the state of polymerization of cell actin might not be as important. A more detailed investigation, dealing with the specific role of small GTPases such as Rho, Cdc42 and also Rac1 and the involvement of critical proteins associated to microtubules and microfilaments, are now beginning to reveal the first differences. These studies are being focused especially on growth cones, the tips of neurites where the exo-endocytotic processes of both outgrowth processes are known to take place ().
A final question is whether the new outgrowth process, dependent on Rac1 and on the exocytosis of enlargesomes, is only a curiosity of defective PC12 cells and other cell lines (for example, the neuroblastoma SH-SY5Y) or can play a physiological role in the nervous system. Neurons are believed to express very low levels of REST/NRSF. Therefore, their enlargeosomes and the Rac-1 dependent outgrowth was expected to be marginal, if anything. Surprisingly, preliminary experiments in embryonal and neonatal neurons, in the tissue and in primary cultures, have revealed the presence of both the enlargeosomes and of new outgrowth process. Ongoing studies of these cells might reveal further surprises. What appears at the moment is that the two outgrowth processes might be important at subsequent studies of neuronal development: the new, enlargeosome-dependent process at early stages; the canonical process later on (). In the past many findings, made first in PC12 cells, have been later confirmed in neurons. This might now be the case of the new outgrowth process. PC12 cells, therefore, are still precious tools for neurobiological research. Discoveries made by their investigation can in fact anticipate and open the way to studies in physiology, with possible consequences also in pathology and medicine.
Figures and Tables
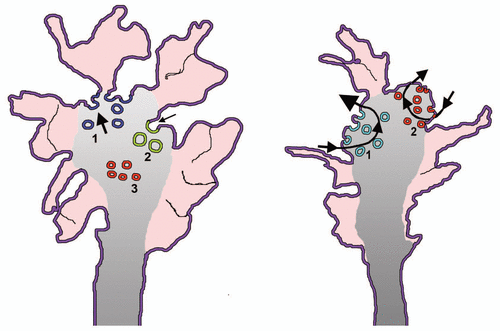
Figure 1 Membrane traffic in growth cones of early and mature neuronal cultures. The model to the left illustrates the structure of growth cones in cultured nerve cells, phase 2 of neurite outgrowth. The external zone labeled in red corresponds to the filopodia and lamellipodia filled with actin (peripheral zone). Vesicles are few in this zone and numerous in the central gray zone, in continuity with the transitional zone. The blue vesicles to the left, labeled 1, are the enlargeosomes undergoing intense exocytosis (thick arrow); the green, large ones to the right, labeled 2, are the vesicles of the bulk endocytosis.Citation18 Notice that their arrow is smaller than that of the exocytic vesicles because a prevalence of exocytosis is necessary for neurites to grow. The small red vesicles labeled 3 are precursors of neurosecretory vesicles, not yet competent for exo/endocytosis. The model to the right illustrates a similar growth cone belonging however to an axon in phase 3/4. Two exo/endocytic cycles are illustrated. That to the left, labeled 1, concerns the TiVAMP-positive vesicles (green) that account for the growth at this stage. The arrow of exocytosis is therefore thicker of that of endocytosis. The cycle to the right, labeled 2, concerns in contrast the small synaptic vesicles (red) and their recycling endocytic vesicles. This cycle does not contribute to the axonal growth, therefore the arrows of exo end endocytosis are of similar thickness.
Addendum to: and
References
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci 1994; 17:267 - 310
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci 1988; 8:1454 - 1468
- Deich JS, Banker GA. An elecron microscopic analysis of hippocampal neurons developing in culture: early stages in the emergence of polarity. J Neurosci 1993; 13:4301 - 4315
- Ledesma MD, Dotti CG. Membrane and cytoskeleton dynamics during axonal elongation and stabilization. Int Rev Cytol 2003; 227:183 - 219
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to NGF. Proc Natl Acad Sci USA 1976; 73:2424 - 2428
- Hang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Ann Rev Biochem 2003; 72:609 - 642
- Pfenninger KH. Plasma membrane expansion: a neuron's herculean task. Nat Neurosci 2009; 10:251 - 261
- Witte H, Bradke F. The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol 2008; 18:479 - 487
- Pfenninger KH, Laurino L, Peretti D, Wang X, Rosso S, Morfini G, et al. Regulation of membrane expansion at the nerve growth cone. J Cell Sci 2003; 116:1209 - 1217
- Martinez-Arca S, Coco S, Mainguy G, Schenk U, Alberts P, Bouillé P, et al. A common exocytotic mechanism mediates axonal and dendritic outgrowth. J Neurosci 2001; 21:3830 - 3838
- Chaineau M, Danglot L, Galli T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett 2009; 583:3817 - 3826
- Chieregatti E, Meldolesi J. Regulated exocytosis: new organelles for non-secretory purposes. Nat Rev Mol Cell Biol 2005; 6:181 - 187
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol 2005; 15:500 - 506
- D'Alessandro R, Klajn A, Stucchi L, Podini P, Malosio ML, Meldolesi J. Expression of the neurosecretory process in PC12 cells is governed by REST. J Neurochem 2008; 105:1369 - 1383
- Leoni C, Menegon A, Benfenati F, Toniolo D, Pennuto M, Valtorta F. Neurite extension occurs in the absence of regulated exocytosis in PC12 subclones. Mol Biol Cell 1999; 10:2919 - 2931
- Borgonovo B, Cocucci E, Racchetti G, Podini P, Bachi A, Meldolesi J. Regulated exocytosis: a novel, widely expressed system. Nat Cell Biol 2002; 4:955 - 962
- Cocucci E, Racchetti G, Rupnik M, Meldolesi J. The regulated exocytosis of enlargeosomes is mediated by a SNARE machinery that includes VAMP4. J Cell Sci 2008; 121:2983 - 2991
- Bonanomi D, Fornasiero EF, Valdez G, Halegoua S, Benfenati F, Menegon A, et al. Identification of a developmentally regulated pathway of membrane retrieval in neuronal growth cones. J Cell Sci 2008; 15:3757 - 3769