Abstract
Recent findings have shown that ubiquitination is involved in regulating several proteins required for cell adhesion and migration. We showed that α5 integrin is ubiquitinated at its cytoplasmic lysines in response to fibronectin binding, and that this is required for its sorting to lysosomes together with fibronectin. Here we speculate whether other α integrin tails may also be ubiquitinated, and discuss the significance of ubiquitin linkages in the regulation of cell adhesion and migration.
Ubiquitination has recently arisen as a regulator of cell adhesion and migration.Citation1 Talin, an integrin-binding protein that is essential for cell migration, is ubiquitinated by the E3 ubiquitin ligase Smurf1, resulting in the proteasomal degradation of its head domain.Citation2 RhoA, which has a role in the assembly of focal adhesions and actin-myosin stress fibers, is also ubiquitinated by Smurf1 and degraded via the proteasome.Citation3 Integrins, which are transmembrane glycoproteins consisting of α and β heterodimers, have also been shown to be regulated by ubiquitination. The platelet integrin αIIbβ3 interacts with the E3 ubiquitin ligase RN181,Citation4 whereas the β integrin subunit in Caenorhabditis elegans is ubiquitinated by an ER-associated E3 ligase, RNF-121.Citation5 Interestingly, while the common thread is ubiquitination, these proteins are not necessarily processed by the same pathways. Such processing is at least in part dependent on the type of ubiquitin linkage (explained below), underlying the importance of the specificity of this posttranslational modification.
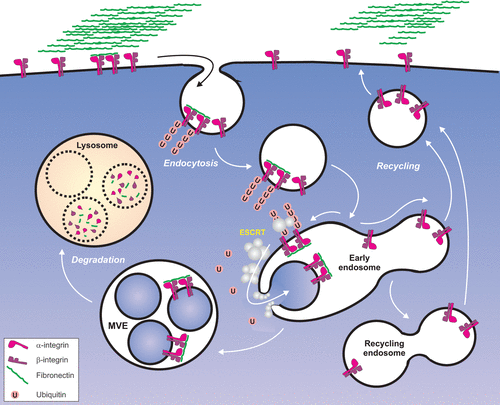
In our studies we have focused on the integrin heterodimer α5β1, which is responsible for cellular interactions with fibronectin matrices. We observed by electron microscopy that a subpopulation of this heterodimer localizes to the lumen of multivesicular endosomes (MVEs), suggesting that it is trafficked there from early endosomes, and on its way to lysosomes ().Citation6 Since ubiquitin acts as a signal to direct trafficking of membrane proteins to lysosomes,Citation7 we investigated whether α5β1 integrin is ubiquitinated. Indeed, both subunits are ubiquitinated. This was observed as a smeared band by SDS-PAGE and western blotting with antibodies against ubiquitin when integrins were immunoprecipitated, suggesting that α5β1 integrin is poly- or multi-monoubiquitinated. Since ubiquitination occurs predominantly at lysine residues, we constructed a mutant where cytoplasmic lysines were substituted with arginines, and we confirmed that ubiquitination was indeed abrogated in the absence of these lysines.
There are several forms of ubiquitination, whose complexity increases since ubiquitin contains seven lysine residues itself. Proteins can be monoubiquitinated on a single lysine residue by a single ubiquitin or multi-monoubiquitinated at several lysine residues. This type of modification has been found to trigger trafficking of membrane proteins to the lysosome, and to be dependent on the endosomal sorting complex required for transport (ESCRT) machinery.Citation8 Since we observed that the ESCRT machinery is required for sorting of integrins into MVEs, it is likely that this type of ubiquitin linkage is conjugated to integrins. However, since we observed smeared bands by western blotting, it is likely that integrins are also polyubiquitinated. Indeed, it has been described that the epidermal growth factor receptor, which is sorted to lysosomes by the ESCRT machinery, is both mono- and polyubiquitinated.Citation9,Citation10 Polyubiquitination can, in principle, occur at any of the seven lysine resides of ubiquitin: K6, K11, K27, K29, K33, K48, K63. Additionally, linear polyubiquitination can occur, in which a C-terminal glycine of an ubiquitin monomer can be conjugated to the N-terminal methionine of the next ubiquitin monomer. To date, the type of ubiquitination of α5-integrin is not known, and in future studies it will be interesting to learn more about this.
In order to consider whether other α-integrins than α5 may undergo ubiquitination, we have compared the cytoplasmic tails of all human α integrins (). Interestingly, we observe that the initial lysine of the cytoplasmic sequence is strictly conserved between all members of the α integrins, with the exception of αv integrin. Furthermore, the next lysine is also conserved between several of the members, if not quite so conservatively. This raises the question whether other α-integrins than α5 are also ubiquitinated, and whether this is the site at which ubiquitination occurs. However, since this lysine is very close to the transmembrane region, one might suppose that the eventual type of polyubiquitination might be Lysine-63 linked or linear-linked, since these are much more linear modifications compared to lysine 48-linked polyubiquitin.
The biological significance of α5 ubiquitination is its sorting to the lysosome for its subsequent degradation. However, we observed a half-life of α5β1 integrin of about 18 hours. It is difficult toreconcile this slow degradation rate with the observation that a substantial fraction of α5 integrin can be found in late endosomes at steady state. One possibility is that the degradation rate is in fact higher than we measured, since we monitored the total pool of integrins, while it would be optimal to measure the activated fibronectin-bound pool of integrins, knowing that integrin and fibronectin are trafficked together. Alternatively, we cannot rule out that integrins may be partially recycled back to the plasma membrane from late endosomes or lysosomes or shed as exosomes, and this might indeed contribute to a slow rate of degradation. It would be interesting to determine whether the integrins are recycled back in their active conformation or not, since the ligand fibronectin is itself degraded, and what their fate is once recycled. For instance, it will be important to learn whether recycled integrins can bind ligand and be re-endocytosed. In such a context, integrins would be trafficked to lysosomes solely to transport fibronectin there, which presumably cannot dissociate from its receptor in endosomes despite the low pH.
In light of these considerations, it needs to be determined what type of ubiquitin linkage is conjugated to integrins, and at which site(s), in order to be able to extend our knowledge to other α integrins. It will also be crucial to identify the E3 ubiquitin ligases that catalyze the ubiquitination of α5 and other mammalian integrins. Given the importance of the interactions between cells and the intracellular matrix in diseases such as cancer, our newly gained understanding of how these are regulated by ubiquitination may offer new avenues for therapeutic intervention, for instance by targeting E3 ubiquitin ligases that are specifically involved in these processes.
Addendum to:
References
- Huang C. Roles of E3 ubiquitin ligases in cell adhesion and migration. Cell Adh Migr 2010; 4:10 - 18
- Huang C, Rajfur Z, Yousefi N, Chen Z, Jacobson K, Ginsberg MH. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat Cell Biol 2009; 11:624 - 630
- Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 2003; 302:1775 - 1779
- Brophy TM, Raab M, Daxecker H, Culligan KG, Lehmann I, Chubb AJ, et al. RN181, a novel ubiquitin E3 ligase that interacts with the KVGFFKR motif of platelet integrin alpha(IIb)beta3. Biochem Biophys Res Commun 2008; 369:1088 - 1093
- Darom A, Bening-Abu-Shach U, Broday L. RNF-121 is an endoplasmic reticulum-membrane E3 ubiquitin ligase involved in the regulation of beta-integrin. Mol Biol Cell 2010; 21:1788 - 1798
- Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerod L, Stenmark H. Ubiquitination of alpha5beta1 Integrin Controls Fibroblast Migration through Lysosomal Degradation of Fibronectin-Integrin Complexes. Dev Cell 2010; 19:148 - 159
- Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 2009; 458:445 - 452
- Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nature Rev Mol Cell Biol 2007; 8:355 - 68
- Galcheva-Gargova Z, Theroux SJ, Davis RJ. The epidermal growth factor receptor is covalently linked to ubiquitin. Oncogene 1995; 11:2649 - 2655
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol 2003; 5:461 - 466