Abstract
Sex pheromones are the hallmark of reproductive behavior in moths. Mature females perform the task of mate signaling and release bouquets of odors that attract conspecific males at long range. The pheromone chemistry follows a relatively minimal design but still the combinatorial action of a handful of specialized pheromone production enzymes has resulted in remarkably diverse sexual signals that subtly vary in structure and in number and ratio of components. In a recent article,1 we showed that a single reductase gene (pgFAR) enables the conversion of key biosynthetic fatty-acyl precursors into fatty alcohols, the immediate precursors of the multi-component pheromone in small ermine moths (Lepidoptera: Yponomeutidae). In the light of the widespread usage of multi-component pheromone blends across Lepidoptera, it is likely that the pgFAR biochemical flexibility is a regular feature of the moth pheromone machinery and polyvalent reductase genes are emerging as pivots to promote phenotypic transitions in moth mating signals. In addition, the small ermine moth pgFAR nevertheless contributes to regulating the ratio among components. Here we show that the pgFAR substrate specificity is actually counterbalancing the inherent chain-length preference of an upstream desaturase with ∆11-activity and that the enzymes together modulate the final blend ratio between the Z11-16:OH, Z11-14:OH and E11-14:OH compounds before the final acetylation.
Moth mate-recognition depends on a sophisticated pheromone-based communication system that allows males to locate calling females over long distances.Citation2,Citation3 The need for a tight coordination between female signalers and recipient males poses selection against variation in sex pheromone composition.Citation2,Citation4,Citation5 The strong stabilizing forces acting to maintain recognition have however not precluded pheromone signals from evolving, as witnessed by the remarkable diversity of more or less species-specific moth pheromones.Citation6 To warrant species-specific interactions, moth sex pheromones typically consist of mixtures of unsaturated (i.e., 1 to 3 double bonds) long-chain fatty alcohols and/or acetates and aldehydes produced de novo from ubiquitous fatty acids.Citation7 How phenotypic transitions in the emitted signals are initiated is intriguing and has raised curiosity about the genetic bases of pheromone production.Citation8 Pheromone biosynthesis involves a small cassette of specialized enzymes including the action of fatty-acyl-CoA desaturases and β-oxidases followed by modifications of the carboxyl group by fatty-acyl-CoA reductases, alcohol oxidases and acetyl transferases.Citation7,Citation9 Gene candidate approaches combined with the use of in vitro heterologous expression systemsCitation10,Citation11 have been successful in enhancing our knowledge about a gene family of fatty-acyl-CoA desaturases. These enzymes exhibit a wide range of desaturase activities (e.g., Δ6, Δ8, Δ9, Δ11, Δ11–12, Δ11–13, Δ14), which enhance structural diversity in moth pheromones.Citation12–Citation14 However, the regulatory mechanisms by which the blend composition is precisely determined are far from being decoded and not all biosynthetic genes controlling moth pheromone production are yet characterized although recent transcriptomic advances have targeted some potential missing candidates.Citation15,Citation16 Recently, a Lepidopteraspecific gene subfamily of fatty-acyl reductases (pgFARs) has been pinpointed that is specifically active in moth pheromone productionCitation1,Citation17,Citation18 and which provides new insights into the evolutionary mechanisms that shaped the lepidopteran mate communication signals.
Evolution of Polyvalent pgFARs
The insect reductase gene family appears to be particularly diverse. In the silkmoth Bombyx mori genome 22 paralogs have been identified that cluster into ten well-supported clades,Citation1 but the function of most homologous FAR genes is still unknown. A single gene subfamily appears Lepidoptera-specificCitation18 and comprises all moth pgFARs identified to date, which encode pheromone gland-specific enzymes functioning as a requisite component of the moth pheromone reduction system.Citation1,Citation17,Citation18 Four B. mori pgFAR-like orthologues exist,Citation1 which suggest that several rounds of duplication events occurred in the active clade although a single pgFAR is required to produce the Bombykol sex pheromone.Citation17 In contrast to the silkmoth, many lepidopteran species including the small ermine mothsCitation19 rely on complex mixtures. Our recent functional investigation of the reduction step enlightens that only one pgFAR gene is active in multicomponent pheromone production in the small ermine moths, but this reductase enzyme displays a broad reductive profile that is adapted to the speciesspecific pheromone composition. Besides, the highly flexible reductase is also capable of processing some rare unsaturated pheromone precursor structures, not found in Yponomeuta spp. These findings suggest that rapidly diversifying desaturation mechanismsCitation11 are more likely to cause quick changes in sex pheromone composition if downstream biosynthetic enzymes are preadapted to process the structural novelties. Taking into account our findings from Yponomeuta spp. together with the widespread use of multi-component pheromones in the Lepidoptera, we propose that the biochemical flexibility of the reduction system may be a widespread and ancient feature of the biosynthetic moth machinery. This preadaptation could have served as a pivot towards a diversification in the signal in the lepidopteran mate communication system, to the extent that changes occur upstream in the pheromone machinery. Comparative studies including other lepidopteran species are awaited to determine whether subclasses exist within the pgFAR subfamily and to improve the phylogenetic resolution with respect to affinities for a certain substrate chain-length (e.g., C14, C16 or C18) and/or variable structures (e.g., mono versus diunsaturated compounds), as previously observed in moth pheromone desaturases.Citation6 Likewise, it will be interesting to see if pgFAR-like duplicates exist in moth species other than B. mori as well as in butterflies and to assess their potential functions in vitro, taking advantage of the fact that we now have developed functional tools for this class of enzymes.
Polygenic Nature of Sex Pheromone Ratio Determination
Because the reduction stage is a requisite step at the interface between fatty-acyl precursors and all derived oxygenated pheromone molecules, it is interesting to uncover the functional basis for selectivity and substrate specificity of pgFARs, yet the underlying genetic determinants have just been discovered. Rare examples are known where single genes (including the reductase system) exclusively control the ratio between components in the pheromone blend.Citation18,Citation20 Still, in many moth species such as Yponomeuta spp., the combined activity of several biosynthetic enzymes is postulated to be required to achieve the desired proportions.Citation2,Citation9,Citation21
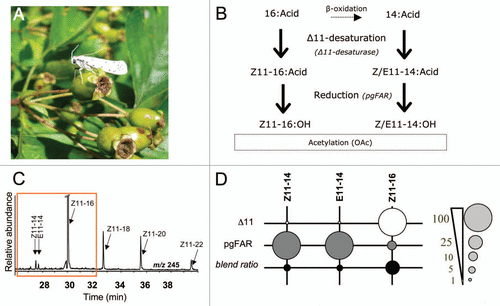
The small ermine moth pgFAR exhibits an inherent preference for Z11-14 and E11-14:acyl substrates compared to Z11-16:acyl. This plays a role in regulating the final proportions between the pheromone components. Hence we showed that when supplying all three Δ11-unsaturated substrates in a ratio biased towards the Z11-16:acyl, i.e., as found in pheromone gland precursors of the orchard ermine moth, Yponomeuta padellus, the outcome of pgFAR conversion matched the final blend proportions. In we show that the bias toward the Z11-16:acyl precursor in the gland results from the action of a Δ11-desaturase with a chain-length preference for C16 acyl. We characterized the corresponding desaturase transcript from cDNA of adult female pheromone gland tissues and subsequently cloned and expressed its encoded protein in a desaturase- and elongase-deficient (ole1 elo1) strain of the yeast Saccharomyces cerevisiae following previously established in vitro techniques.Citation10,Citation14 We found that the enzyme displays a Δ11-desaturase activity resulting in the preferential production of Z11-16:acyl compared to Z11- and E11-14:acyls (). The succeeding action of the pgFAR, which exhibits a chainlength preference for C14-acyls, balances the desaturase effects and adjusts the ratio between all Δ11-alcohol precursors in proportions matching the final blend observed in females. The activity of both Δ11 and pgFAR proteins thus regulates the proportions of Δ11-pheromone precursors in this species. In order to gain a complete understanding of the proximate genetic mechanisms driving the evolution of multi-component signals in moths, the next challenge will be to unravel the potential role of acetyl transferase gene products in adjusting the relative conversion of alcohols and acetates in sister Yponomeuta species but also in other moths.Citation22 Further research in this direction will provide openings to co-express all genetic components of the biosynthetic machinery and produce sex pheromones in vitro, which we envision as an alternative strategy to produce synthetic sex pheromones.
Abbreviations
| pgFAR | = | pheromone-gland specific fatty-acyl CoA reductase |
| OH | = | alcohol |
| OAc | = | acetate |
Figures and Tables
Figure 1 Pheromone biosynthesis in Yponomeuta padellus (Lepidoptera: Yponomeutidae). (A) An Yponomeuta moth on hawthorn (Crataegus sp., Rosaceae). (B) Pheromone biosynthetic pathway towards the Δ11-unsaturated components in the Yponomeuta genus. (C) GC-MS analysis of Δ11-monounsaturated fatty-acyl intermediates produced by functional expression of the Ypa-Δ11-desaturase in the YEpOLEX expression vector and the ole 1 elo 1 strain of the yeast Saccharomyces cerevisiae following a procedure as previously described.Citation14 The chromatogram trace represents dimethyl disulfide adducts (DMDS) from methanolyzed yeast extracts. In vitro, the Δ11-desaturase catalyses the introduction of a double bond at the eleventh carbon atom (characteristic ion at m/z 245) in several natural yeast fatty acids from C14 to C22. The Z11-14, E11-14 and Z11-16 acyls are produced in a 1.1:0.8:100 ratio, respectively. (D) The dot areas are proportional (%) to the Δ11-desaturase and pgFAR substrate preferences and to the final ratio between components. The reverse chain-length preference of the Δ11-desaturase and pgFAR for acyl substrates with 14 or 16 carbon atoms allows adjusting the final blend ratio.
Addendum to:
References
- Liénard MA, Hagström ÅK, Lassance JM, Löfstedt C. Evolution of multi-component pheromone signals in small ermine moths involves a single fatty-acyl reductase gene. Proc Natl Acad Sci USA 2010; 107:10955 - 10960
- Cardé RT, Haynes KF. Cardé RT, Millar JG. Advances in Insect Chemical Ecology 2004; Cambridge University Press 283 - 332
- Wyatt TD. Pheromones and animal behaviour. Communication by smell and taste 2004; Cambridge University Press
- Löfstedt C. Moth pheromone genetics and evolution. Phil Trans R Soc Lond B 1993; 340:167 - 177
- Phelan PL. Choe JC, Crespi BJ. The evolution of mating systems in insects and arachnids 1997; University Press 240 - 256
- Ando T, Inomata SI, Yamamoto M. Lepidopteran sex pheromones. Top Curr Chem 2004; 239:51 - 96
- Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ. Insect pheromones-an overview of biosynthesis and endocrine regulation. Insect Biochem Mol Biol 1999; 29:481 - 514
- Symonds MRE, Elgar MA. The evolution of pheromone diversity. Trends Ecol Evol 2008; 23:220 - 228
- Blomquist GJ, Jurenka RA, Schal C, Tittiger C. Gilbert LI, Iatrou K, Gill S, Elsevier. Comprehensive molecular insect science 2005; 3:Academic 705 - 751
- Knipple DC, Rosenfield CL, Miller SJ, Liu W, Tang J, Ma PWK, et al. Cloning and functional expression of a cDNA encoding a pheromone gland-specific acyl-CoA delta11-desaturase of the cabbage looper moth, Trichoplusia ni. Proc Natl Acad Sci USA 1998; 95:15287 - 15292
- Roelofs WL, Liu W, Hao G, Jiao H, Rooney AP, Linn CEJ. Evolution of moth sex pheromones via ancestral genes. Proc Natl Acad Sci USA 2002; 99:13621 - 13626
- Knipple DC, Rosenfield CL, Nielsen R, You KM, Jeong SE. Evolution of the integral membrane desaturase gene family in moths and flies. Genetics 2002; 162:1737 - 1752
- Roelofs WL, Rooney AP. Molecular genetics and evolution of pheromone biosynthesis in Lepidoptera. Proc Natl Acad Sci USA 2003; 100:9179 - 9184
- Liénard MA, Strandh M, Hedenström E, Johansson T, Löfstedt C. Key biosynthetic gene subfamily recruited for pheromone production prior to the extensive radiation of Lepidoptera. BMC Evol Biol 2008; 8:270
- Strandh M, Johansson T, Ahrén D, Löfstedt C. Transcriptional analysis of the pheromone gland of the turnip moth, Agrotis segetum (Noctuidae), reveals candidate genes involved in pheromone production. Insect Mol Biol 2008; 17:73 - 85
- Vogel H, Heidel AJ, Heckel DG, Groot AT. Transcriptome analysis of the sex pheromone gland of the noctuid moth Heliothis virescens. BMC Genomics 2010; 29:11
- Moto K, Yoshiga T, Yamamoto M, Takahashi S, Okano K, Ando T, et al. Pheromone gland-specific fatty-acyl reductase of the silkmoth, Bombyx mori. Proc Natl Acad Sci USA 2003; 100:9156 - 9161
- Lassance JM, Groot AT, Liénard MA, Antony B, Borgwart C, Heckel DG, et al. Allelic variation in a fatty-acyl reductase gene causes divergence in moth sex pheromones. Nature 2010; 466:486 - 489
- Löfstedt C, Herrebout W, Menken S. Sex pheromones and their potential role in the evolution of sex reproductive isolation in small ermine moths (Yponomeutidae). Chemoecology 1991; 2:20 - 28
- Roelofs WL, Glover T, Tang X, Sreng I, Robbins P, Eckenrode C, et al. Sex pheromone production and perception in European corn borer moths is determined by both autosomal and sex-linked genes. Proc Natl Acad Sci USA 1987; 84:7585 - 7589
- Jurenka RA. Vogt G, Blomquist R. Insect Pheromone Biochemistry and Molecular Biology 2003; New York Academic Press 53 - 80
- Jurenka RA, Roelofs WL. Characterization of the acetyltransferase used in pheromone biosynthesis in moths: specificity for the Z isomer in tortricidae. Insect Biochem 1989; 19:639 - 644