Abstract
Defective autophagy and lysosomal degradation are hallmarks of numerous neurodegenerative disorders. Vesicular ATPases are intracellular proton pumps that acidify autophagosomes and lysosomes. V0a1 is a key component of the v-ATPase that is only required in neurons in Drosophila melanogaster. We have recently shown that loss of V0a1 in Drosophila photoreceptor neurons leads to slow, adult-onset degeneration.1 Concurrently, Lee et al.2 reported that V0a1 fails to localize to lysosomal compartments in cells from Presenilin 1 knock-out cells. Together these two reports suggest that a neuronal V0a1-dependent degradation mechanism may be causally linked to Alzheimer pathology. Indeed, we now show that loss of V0a1 makes Drosophila neurons more susceptible to insult with human Alzheimer-related neurotoxic Aβ and tau proteins. Furthermore, we discuss the potential significance of the discovery of the neuron-specific degradation mechanism in Drosophila for intracellular degradation defects in Alzheimer Disease.
Until recently, the degradation machinery implicated in neuronal degeneration was thought to be ubiquitous and there was little evidence for a dedicated neuronal pathway. In addition, the causal relationship between adult-onset neurodegeneration and accumulations of undegraded proteins in Alzheimer Disease as well as other neurodegenerative disorders is unclear.Citation3,Citation4 We have recently shown that loss of the v-ATPase component V0a1 leads to a ∼50% reduction of strongly acidified compartments in neurons, an increase of undegraded endosomal and autophagosomal compartments, and ultimately to slow, adult-onset degeneration.Citation1 Furthermore, loss of v0a1 also causes severe neurotransmission defects and thereby embryonic lethality. The recent Drosophila studies were made possible by a genetic method that only renders photoreceptor neurons homozygous mutant in otherwise heterozygous animals.Citation5 Since photoreceptors are not required for viability, this system allows one to overcome early neuronal death due to embryonic lethality and follow the maintenance and degeneration of homozygous mutant neurons in an otherwise healthy fly. v0a1 photoreceptors exhibit loss of neurotransmission immediately after eclosion,Citation6 whereas significant functional and morphological neurodegeneration occurs only after 1–2 weeks.Citation1 Importantly, v0a1 is only required in neurons in Drosophila.Citation6 The discovery of a neuron-specific degradation mechanism in Drosophila establishes a causal link between intracellular degradation and adult-onset degeneration. Similarly, disruption of basal autophagy in mice through either loss of atg5 or atg7 leads to neurodegeneration within weeks,Citation7,Citation8 and induction of autophagy can alleviate neurodegenerative effects.Citation9,Citation10 Lysosomal storage disorders show how mutants in endolysosomal and autophagic pathways lead to late onset neurodegeneration.Citation11 Finally, neurons in mouse models of Down Syndrome and Alzheimer Disease exhibit early accumulations of Rab5-marked early endosomes as well as late endosomes.Citation3 All these findings are consistent with the idea that loss of degradation capacity sensitizes neurons to ‘cargo overload’ as a cause for slow, adult-onset degeneration.
Lee et al.Citation2 recently reported that mislocalization of V0a1 is at least partially responsible for causing degenerative phenotypes in Presenilin 1 (PS1) knock-out cells. This finding is consistent with our discovery of the V0a1-dependent neuronal degradation mechanism in Drosophila and the degenerative phenotypes caused by loss of v0a1. The Drosophila, mouse and human V0a1 genes are precise orthologs within the V0a gene families in each of these different species that comprise exactly four V0a1-4 genes. Since loss of v0a1 in Drosophila causes embryonic lethality due to neurotransmission failure, and neurotransmission has been shown to be preserved in PS1 KO neurons,Citation12 we conclude that the trafficking defect of V0a1 reported in PS1 KO cells should not represent a full v0a1 loss-of-function. Instead, we propose that in PS1 KO cells only the trafficking to a subset of degradative compartments is affected. Furthermore, since lethality due to loss of neurotransmission precedes and masks slow degeneration, it may be unlikely to find disease-related v0a1 mutations in humans. In contrast, loss of Presenilin 1 may cause a restricted loss of V0a1 in a specialized degradation pathway.
We were intrigued by the possibility that loss of v0a1-dependent degradation renders neurons more susceptible to neurotoxic insults. To directly test the idea that neurons lacking the neuron-specific degradation pathway are more susceptible to neurotoxic proteins, we designed challenge experiments with human AβCitation13 and tauCitation14 variants. Aβ peptides are the neurotoxic cleavage products of Amyloid Precursor Proteins (APP) that are causally associated with Alzheimer Disease and may have neurotoxic effects by disrupting lysosomal function.Citation15 Of the two possible Aβ cleavage products of APP, Aβ42 is more toxic and its expression in Drosophila photoreceptors leads to early cell death, whereas expression of Aβ40 has much milder phenotypes.Citation13 We established a protocol using Aβ40 expression and v0a1 RNAi expression in addition to two days intense light stimulation; under these conditions, neither Aβ40 nor v100 RNAi cause significant degeneration as measured by ERG response amplitudes ( and B). Knock-down of v0a1 using RNAi causes the same phenotypes as loss of v100 in photoreceptors (), and it provides a sensitized system for degradative challenges, as shown below. Indeed, when we co-express v0a1 RNAi and Aβ40 under these conditions, ERG response amplitudes are significantly reduced compared to both Aβ40 or v100 RNAi alone ( and B). Next, we expressed the human microtubule-binding protein tau as well as a mutant version of tau (R406W) that has been implicated in the pathogenesis of Alzheimer's disease and related disorders.Citation14 Similar to Aβ40, expression of tau and tauR406W lead to increased functional degeneration when co-expressed with v0a1 RNAi ( and B). Morphologically, two day intense light stimulation already leads to a few minor disruptions of the photoreceptor arrangement in the eye that are apparent as ‘holes’, i.e., unlabeled spaces between the rhabdomeric structures (). These aberrations are increased by v100 RNAi expression and indicative of early stages of photoreceptor degeneration. Co-expression of either Aβ40 or tau leads to a dramatic acceleration of this degenerative phenotype (). These results support the idea that loss of v0a1-dependent degradation increases neuronal sensitivity to neurotoxic insults and accelerates neurodegeneration. Taken together with the mouse studies, loss of Presenilin 1 may cause a double burden by decreasing the degradative capacity through mislocalization of V0a1 and increasing the degradative load through faulty APP cleavage, Aβ accumulations or tau tangles.
Does V0a1 define a neuron-specific degradation pathway? In Drosophila, V0a1 is only required in neurons which lead to the idea of a neuron-specific mechanism.Citation1 In contrast, Lee et al.Citation2 characterize a V0a1dependent acidification defect in PS1 KO mouse blastocysts. Degradative compartments require acidification for the maturation of Cathepsin proteases. PS1 KO blastocysts have a defect in Cathepsin D maturation, although a similar defect has not yet been demonstrated for neurons.Citation2,Citation16 Furthermore, Presenilin double knock-out hippocampal neurons have significant ER calcium signaling abnormalities which may also potentially be linked to defects in V0a1 trafficking and impaired acidification of intracellular compartments.Citation17 Overall, the data from Lee et al.Citation2 suggest that a degradation mechanism that is neuron-specific in Drosophila may also operate in non-neuronal cells in mammals. Indeed, expression data inferred from Expressed Sequence Tag sources available from the public database of the National Center for Biotechnology Information (NCBI) indicates that v0a1 expression is not restricted to neurons in mouse and human. Interestingly, the observation of neuronal specificity for a protein that is more widely expressed in mammals has also been made for APPL, the Drosophila ortholog of the Aβ-precursor protein APP. While expression of Drosophila APPL is a restricted to the nervous system,Citation18,Citation19 two of the three APP homologs in the mouse are ubiquitously expressed.Citation20 However, the recent findings in v0a1 Drosophila neurons and PS1 mouse KO cells suggest that v0a1 is not required for all intracellular degradation. Instead, V0a1 increases degradative capacity and its loss alone is sufficient to cause adult-onset degeneration in Drosophila neurons. In addition, loss of V0a1-dependent degradation makes cells more vulnerable to neurotoxic insults including Aβ and tau proteins. Further experiments will be needed to validate the contribution of the v0a1-dependent degradation mechanism to neurodegenerative pathology.
Materials and Methods
Experiments were conducted using flies of the genotype UAS-Dicer2;GMR-Gal4;UAS-v0a1-RNAi and additionally expressing either UAS-Aβ40, UAS-tau or UAS-tau406. The v0a1 RNAi line was obtained from the VDRC.Citation21 Electroretinogram recordings, immunohistochemistry and quantification were all conducted as described in Williamson et al.Citation1 Dissections were performed as described in Williamson and Hiesinger.Citation22 For immunohistochemistry, the following antibodies and dilutions were used: Chaoptin (mAb 24B10) at 1:50, Avalanche/Syx7 at 1:1,000 and Sunglasses at 1:1,000.
Figures and Tables
Figure 1 Loss of the v0a1-dependent degradation pathway increases susceptibility to neurotoxic insults by human Aβ and tau proteins. (A) Representative ERG recording for at least n = 20 three day-old flies of the genotypes indicated and raised at room temperature under constant light stimulation. (B) Quantification of ERG depolarizations. Orange bar shows ERG depolarization for control or overexpressed proteins in wild type photoreceptors; blue bars are corresponding experiments with dicer2-enhanced v0a1 rNAi. Asterisks show statistical significance in pairwise comparisons (p < 0.005). (C) Immunolabeled adult eyes from experimental flies used for ERG recordings shown in (A). Chaoptin in green and nuclear labeling with Toto-3 in blue; Syx7/Avl labeling in red. Arrows indicate degenerative ‘holes’ in the immunolabeling. Arrowheads indicate early endosomal Syx7-positive accumulations with Chaoptin that are typical for loss of v0a1 function. Error bars are S.E.M.
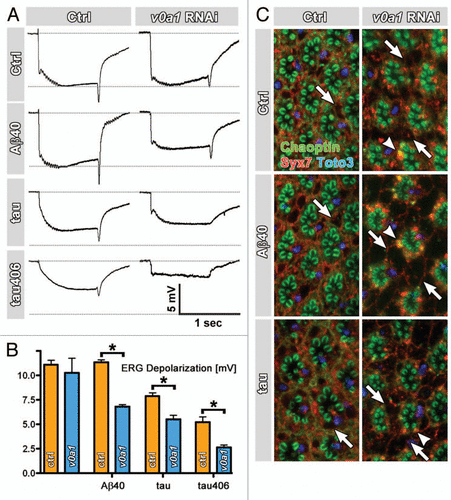
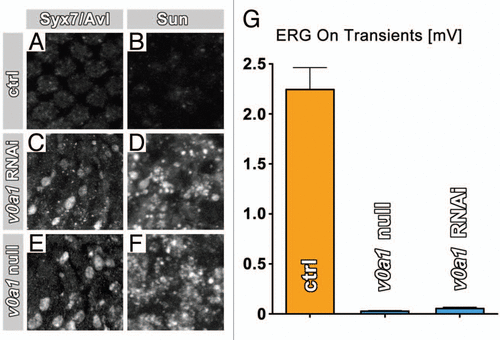
Figure 2 v0a1 rNAi in photoreceptors causes phenotypes identical to loss of v0a1. (A–F) Antibody-labeling of Syx7/Avl and Sunglasses reveals similar accumulations of both markers in photoreceptors expressing dicer2-enhanced v0a1 RNAi (C and D) as in v0a1null mutant photoreceptors in ey35FLP v0a1 flies (E and F). (G) Both v0a1 RNAi as well as ey35FLP v0a1 lead to a complete loss of neurotransmission, as indicated by the loss of electroretinogram (ERG) ‘on’ transients.
Acknowledgements
We would like to thank Drs. Helmut Kramer, Damian Crowther, Craig Montell, the Bloomington Stock Center, and the University of Iowa Developmental Studies Hybridoma Bank for reagents. We further thank Drs. Ilya Bezprozvanny and Adam Haberman for comments on the manuscript and discussion. This work was supported by grants from the National Institute of Health (RO1EY018884) and the Welch Foundation (I-1657). P.R.H. is a Eugene McDermott Scholar in Biomedical Research.
Addendum to:
References
- Williamson WR, Wang D, Haberman AS, Hiesinger PR. A dual function of V0-ATPase a1 provides an endolysosomal degradation mechanism in Drosophila photoreceptors. J Cell Biol 2010;
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 2010; 141:1146 - 1158
- Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy 2008; 4:590 - 599
- Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 2010; 13:805 - 811
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 2000; 127:851 - 860
- Hiesinger PR, Fayyazuddin A, Mehta SQ, Rosenmund T, Schulze KL, Zhai RG, et al. The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 2005; 121:607 - 620
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006; 441:880 - 884
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006; 441:885 - 889
- Wang T, Lao U, Edgar BA. TOR-mediated autophagy regulates cell death in Drosophila neurodegenerative disease. J Cell Biol 2009; 186:703 - 711
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 2008; 451:1069 - 1075
- Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol 2004; 5:554 - 565
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 2001; 32:911 - 926
- Crowther DC, Kinghorn KJ, Miranda E, Page R, Curry JA, Duthie FA, et al. Intraneuronal Abeta, non-amyloid aggregates and neurodegeneration in a Drosophila model of Alzheimer's disease. Neuroscience 2005; 132:123 - 135
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, et al. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 2001; 293:711 - 714
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci 2007; 8:499 - 509
- Esselens C, Oorschot V, Baert V, Raemaekers T, Spittaels K, Serneels L, et al. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J Cell Biol 2004; 166:1041 - 1054
- Zhang H, Sun S, Herreman A, De Strooper B, Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. J Neurosci 30:8566 - 8580
- Martin-Morris LE, White K. The Drosophila transcript encoded by the beta-amyloid protein precursor-like gene is restricted to the nervous system. Development 1990; 110:185 - 195
- Leyssen M, Ayaz D, Hebert SS, Reeve S, De Strooper B, Hassan BA. Amyloid precursor protein promotes post-developmental neurite arborization in the Drosophila brain. EMBO J 2005; 24:2944 - 2955
- Slunt HH, Thinakaran G, Von Koch C, Lo AC, Tanzi RE, Sisodia SS. Expression of a ubiquitous, cross-reactive homologue of the mouse beta-amyloid precursor protein (APP). J Biol Chem 1994; 269:2637 - 2644
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007; 448:151 - 156
- Williamson WR, Hiesinger PR. Preparation of developing and adult Drosophila brains and retinae for live imaging. J Vis Exp 2010;