Abstract
Utilizing the human monocyte/macrophage cell line THP1, we recently identified extracellular ubiquitin as an endogenous agonist of the G protein-coupled receptor CXC chemokine receptor (CXCR) 4. Because receptor binding and signaling properties of extracellular ubiquitin have not been evaluated in primary human leukocytes, we analyzed its binding characteristics and subsequent Ca2+ signaling in freshly isolated human B-cells, T-cells and monocytes. Ubiquitin binding shows typical receptor binding characteristics and promotes intracellular Ca2+ flux within seconds in all three cell populations. The Kd for the ubiquitin receptor interaction in freshly isolated human monocytes is consistent with the affinity of the ubiquitin CXCR4 interaction that we reported for THP1 cells. As detected in THP1 cells previously, the ubiquitin induced Ca2+ flux can be attenuated with a phospholipase C inhibitor in all primary leukocyte cultures. Our observations further support the finding that ubiquitin is a CXCR4 agonist and demonstrate that extracellular ubiquitin induces physiological relevant signaling events in primary human leukocytes. Although the exact mechanism of the ubiquitin CXCR4 interaction, its receptor selectivity and subsequent signaling events remain to be determined, our findings identify a novel and unexpected biological role of extracellular ubiquitin as an endogenous immune modulator.
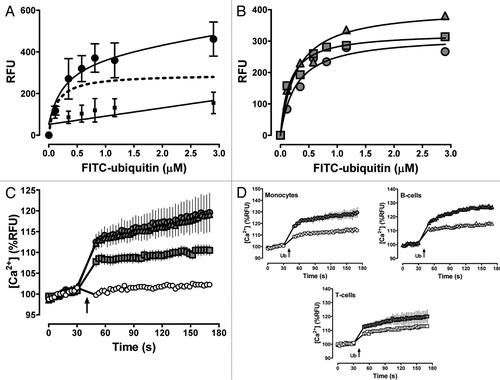
Ubiquitin is a post-translational protein modifier in all eukaryotic cells.Citation1 Besides its intracellular localization and function, ubiquitin has also been detected in various extracellular fluids, such as plasma, cerebrospinal fluid, bronchoalveolar lavage fluid, seminal plasma, amniotic fluid or urine and increased levels of extracellular ubiquitin have been described in a variety of diseases.Citation2 Multiple lines of evidence suggest that extracellular ubiquitin has pleiotropic functions in the innate immune system and that administration of exogenous ubiquitin attenuates inflammation and reduces organ injury in several disease models.Citation2 However, its mechanism of action remained unknown. Utilizing the human acute monocytic leukemia cell line THP1, we recently identified ubiquitin as an agonist of the G protein-coupled receptor (GPCR) CXC chemokine receptor (CXCR) 4.3 Although we demonstrated by flow cytometry that N-terminal fluorescein labeled ubiquitin (FITC-ubiquitin) binds to various human and murine monocyte/macrophage cell lines and freshly isolated human monocytes at 4°C,Citation3 the receptor binding and signaling properties of ubiquitin have not been evaluated in primary human leukocytes. To confirm that ubiquitin binding to the cell surface of primary human monocytes shows also typical receptor binding characteristics, we isolated monocytes from freshly prepared buffy coats by density gradient centrifugation, followed by plastic adherence.Citation3 Buffy coats from healthy blood donors were obtained from Lifesource, Chicagoland's blood center. Ubiquitin receptor binding was tested after incubation of the cells with FITC-ubiquitin (Boston Biochem) for 1 min at 4°C, as described.Citation3 Reactions were performed in the presence of 1% bovine serum albumin to prevent nonspecific binding. As shown in , FITC-ubiquitin binding to monocytes was saturable and could be prevented by an excess of unlabeled ubiquitin. Based on the specific FITC-ubiquitin binding curve from experiments with monocytes obtained from seven blood donors, the Kd was 130 ± 60 nM in saturation binding experiments. This affinity of the ubiquitin receptor interaction is comparable with its receptor affinity that we determined previously in THP1 cells.Citation3 As CXCR4 is abundantly expressed on lymphocytes,Citation4 we also performed initial experiments with B and T cells. Pan B cells, T cells and monocytes were isolated from PBMCs via negative selection using an indirect magnetic labeling system (MACS LS, Miltenyi Biotech Inc., CA). shows typical specific receptor binding curves with leukocytes from a single donor. Consistent with the cell surface expression of CXCR4, ubiquitin receptor binding was detectable in all three cell types. In agreement with a higher expression of CXCR4 on the cell surface of B-cells,Citation5 the Bmax value that we determined in B-cells was higher than the Bmax values determined for T-cells and monocytes (RFU; B-cells: 406 ± 30; T-cells: 317 ± 26; monocytes: 326 ± 16; p = 0.042). As CXCR4 activation promotes intracellular Ca2+ flux, we further confirmed that ubiquitin possess biological activity in primary human leukocytes using the Fluo-4NW calcium assay (Molecular Probes).Citation3,Citation6 shows the results of the Ca2+ flux measurements with the leukocyte preparations that we used in . As expected for a CXCR4 agonist, addition of ubiquitin to the cell cultures induced intracellular Ca2+ flux within seconds. Consistent with our observations in THP1 cells, the phospholipase C (PLC) inhibitor U73122 was able to inhibit ubiquitin induced Ca2+ flux in all cell cultures, as compared with the weak PLC inhibitor U73343 (both from EMD Biosciences). These additional data further support our finding that ubiquitin is a CXCR4 agonist and demonstrate that it induces physiological relevant signaling events in primary human leukocytes.
Chemokine receptors and their ligands are highly promiscuous, being able to bind multiple receptors/ligands.Citation6,Citation7 While stromal-cell derived factor (SDF)-1α ((CX-C motif) ligand (CXCL) 12) has been shown to bind to CXCR4 and CXCR7, known endogenous CXCR4 ligands are SDF-1α, macrophage migration inhibitory factor (MIF),Citation6–Citation9 and now ubiquitin.Citation3 Because of the promiscuity of chemokine receptors, it appears possible that ubiquitin binds to multiple receptors of this family. Depending on the specific agonist, a single GPCR can signal through different pathways with different efficacies, which is known as biased agonism or functional selectivity.Citation10–Citation12 Biased agonism has been suggested for CXCR4 by findings which imply the presence of alternative agonist-binding sites.Citation13 Although previously reported in vivo and in vitro effects of ubiquitin and SDF-1α are consistent with CXCR4 as their common recep tor,Citation14–Citation26 it cannot be excluded that ubiquitin and SDF-1α activation of CXCR4 evoke distinct biological effects. While the precise mechanism of CXCR4 activation by ubiquitin as well as the subsequent intracellular signaling events remain to be determined, our findings identify a novel biological role of ubiquitin when it is released into the extracellular space. Because ubiquitin is one of the most highly conserved proteins in all eukaryotes,Citation27 its anti-inflammatory actions could constitute a primordial anti-inflammatory mechanism that is conserved throughout the phylogenetic system.
Figures and Tables
Figure 1 (A) FITC-ubiquitin binding to human monocytes (1 min, 4°C). Note that cells were centrifuged for 5 min to remove free FITC-ubiquitin in the cell culture supernatant. Data are mean ± Sem of duplicate measurements with monocytes from seven healthy blood donors. ●, FITC-ubiquitin binding; ■, non-specific binding, as assessed by binding of FITC-ubiquitin in the presence of 300 µm native ubiquitin; dashed line, specific binding curve (= total FITC-ubiquitin binding - non-specific binding; r2: 0.93). (B) Specific FITC-ubiquitin binding curves in monocytes (), B () and t cells () from a single blood donor, determined as in (A). (C) ubiquitin (3 µm) induced Ca2+ flux in monocytes (![]()
Addendum to:
References
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998; 67:425 - 479
- Majetschak M. Extracellular ubiquitin: immune modulator and endogenous opponent of damage-associated molecular pattern molecules. J Leukoc Biol 2010; In press
- Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem 2010; 285:15566 - 15576
- Gupta SK, Pillarisetti K. Cutting edge: CXCR4-Lo: molecular cloning and functional expression of a novel human CXCR4 splice variant. J Immunol 1999; 163:2368 - 2372
- Duchesneau P, Gallagher E, Walcheck B, Waddell TK. Upregulation of leukocyte CXCR4 expression by sulfatide: an L-selectin-dependent pathway on CD4+ T cells. Eur J Immunol 2007; 37:2949 - 2960
- Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta 2007; 1768:952 - 963
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, et al. International union of pharmacology. Nomenclature for chemokine receptors. Pharmacol Rev 2000; 52:145 - 176
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 2007; 13:587 - 596
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 2005; 280:35760 - 35766
- Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol 2010; 80:1 - 12
- Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem 2008; 283:5669 - 5676
- Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov 2010; 9:373 - 386
- Sachpatzidis A, Benton BK, Manfredi JP, Wang H, Hamilton A, Dohlman HG, et al. Identification of allosteric peptide agonists of CXCR4. J Biol Chem 2003; 278:896 - 907
- Majetschak M, Krehmeier U, Bardenheuer M, Denz C, Quintel M, Voggenreiter G, et al. Extracellular ubiquitin inhibits the TNFalpha response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood 2003; 101:1882 - 1890
- Garcia-Covarrubias L, Manning EW 3rd, Sorell LT, Pham SM, Majetschak M. Ubiquitin enhances the Th2 cytokine response and attenuates ischemiareperfusion injury in the lung. Crit Care Med 2008; 36:979 - 982
- Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N. CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med 2008; 205:2643 - 2655
- Zheng H, Dai T, Zhou B, Zhu J, Huang H, Wang M, et al. SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis 2008; 201:36 - 42
- Shyu WC, Lin SZ, Yen PS, Su CY, Chen DC, Wang HJ, et al. Stromal cell-derived factor-1alpha promotes neuroprotection, angiogenesis and mobilization/homing of bone marrow-derived cells in stroke rats. J Pharmacol Exp Ther 2008; 324:834 - 849
- Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, et al. Stromal cell derived factor-1alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1alpha CXCR4 axis. Circulation 2007; 116:654 - 663
- Majetschak M, Cohn SM, Nelson JA, Burton EH, Obertacke U, Proctor KG. Effects of exogenous ubiquitin in lethal endotoxemia. Surgery 2004; 135:536 - 543
- Majetschak M, Cohn SM, Obertacke U, Proctor KG. Therapeutic potential of exogenous ubiquitin during resuscitation from severe trauma. J Trauma 2004; 56:991 - 999
- Earle SA, Proctor KG, Patel MB, Majetschak M. Ubiquitin reduces fluid shifts after traumatic brain injury. Surgery 2005; 138:431 - 438
- Griebenow M, Casalis P, Woiciechowsky C, Majetschak M, Thomale UW. Ubiquitin reduces contusion volume after controlled cortical impact injury in rats. J Neurotrauma 2007; 24:1529 - 1535
- Earle SA, El-Haddad A, Patel MB, Ruiz P, Pham SM, Majetschak M. Prolongation of skin graft survival by exogenous ubiquitin. Transplantation 2006; 82:1544 - 1546
- Ahn HC, Yoo KY, Hwang IK, Cho JH, Lee CH, Choi JH, et al. Ischemia-related changes in naive and mutant forms of ubiquitin and neuroprotective effects of ubiquitin in the hippocampus following experimental transient ischemic damage. Exp Neurol 2009; 220:120 - 132
- Singh M, Roginskaya M, Dalal S, Menon B, Kaverina E, Boluyt MO, et al. Extracellular ubiquitin inhibits beta-AR-stimulated apoptosis in cardiac myocytes: role of GSK-3beta and mitochondrial pathways. Cardiovasc Res 2010; 86:20 - 28
- Ozkaynak E, Finley D, Varshavsky A. The yeast ubiquitin gene: head-to-tail repeats encoding a polyubiquitin precursor protein. Nature 1984; 312:663 - 666