Abstract
CD44 is a widely-expressed adhesion receptor that is associated with diverse biological processes involving migrating cells, including inflammation, angiogenesis, bone metabolism, and wound healing. In the immune system, CD44 is upregulated after activation of naive T lymphocytes during their responses against invading microbes. Once an infection is cleared, elevated levels of CD44 remain on the surface of memory T cells that mediate protection against re-infection. While this has led to the use of highly sustained CD44 expression on T cells as an indicator of a previous immune response, the relevance to T-cell responses or homeostasis has been largely unexplored. Our recent studies demonstrate that CD44 selectively regulates the survival of the Th1 subset of CD4 T cells, but not other T-cell subpopulations. These findings, together with studies of CD44 in other cell types, suggest that differences in the engagement of signaling mechanisms are likely to underlie differential regulation of T-cell responses and underscore the importance of this adhesion receptor to immune cell regulation and protection against viruses and intracellular bacteria.
Introduction
CD44 is a type I transmembrane glycoprotein with a C-type lectin domain in the N-terminal domain that binds the major in vivo ligand, hyaluronic acid (HA), a glycosaminoglycan component of the extracellular matrix (ECM) involved in maintaining tissue integrity.Citation1 Encoded by a single gene containing 21 exons, Cd44 gives rise to a family of molecules with an invariant form and at least 5 isoforms generated by alternative mRNA splicing. Although all forms of CD44 bind HA, the affinity is regulated by post-translational modifications such as glycosylation and sialylation.Citation2,Citation3 Most cells, including resting T cells, express the invariant form of CD44. We have not detected CD44 variants on murine CD4 T-cell subsets, including Th1, Th2 or Th17 cells.Citation4 However, isoform expression is observed on T cells that infiltrate target tissues in various autoimmune and inflammatory diseasesCitation5,Citation6 and transient expression of CD44 variants can occur.Citation7 CD44 can be activated from a low to high affinity state on T cells by HA-binding itself,Citation8,Citation9 TCR engagement,Citation8 and responses to cytokines/chemokines.Citation10 Furthermore, T cell-derived IL-2, TNFα and IFNγ can regulate HA binding to monocyte CD44, highlighting the potential indirect effects that T cells may have on CD44 function on other cell types.Citation11,Citation12 In this review, the consequences of CD44 engagement on T-cell migration and signaling will be discussed specifically.
The Role of CD44 in T-Cell Migration
CD44 has been best characterized as an adhesion receptor engaged by migrating T cells. On activated T cells, CD44 can regulate tethering and rolling interactions with vascular endothelial cells that express HA.Citation13 These findings demonstrate that CD44 could provide an alternative to usage of the selectin family of C-type lectins in initiating the multistep adhesion cascade that leads to the extravasation of lymphocytes from the blood via the endothelium into tissues. The CD44-dependent adhesion mechanism is likely to be most important for the mobilization of effector T cells at sites of infection and inflammation because expression of CD44 is upregulated whereas L-selectin (CD62L), the lymphocyte-expressed selectin, is downregulated. In vitro, CD44 associates with the integrin VLA-4 (α4/β1; CD49d/CD29) in the membrane of activated T cells through its cytoplasmic tail, and in vivo this receptor combination can regulate T-cell extravasation into the peritoneum after induced inflammation.Citation14,Citation15 The association between the two surface receptors thereby not only allows for firm adhesion, but also could provide each with access to the other's signaling pathways, which would otherwise be unavailable. Deletion of the cytoplasmic tail of CD44 prevents firm adhesion of cells to the endothelium, highlighting the importance of the molecules' association for T-cell extravasation. The VLA-4-ligand, VCAM-1, is induced on endothelial cells in response to inflammatory cytokines and HA synthesis and expression are also augmented. However, CD44-dependent tethering/rolling could also occur independently of HA via binding to E-selectin, which is expressed on activated endothelium.Citation16 However, we and others have shown that CD44-independent mechanisms can also regulate effector T-cell recruitment to sites of inflammation or tumor implantation because CD44-deficiency does not impair migration.Citation4,Citation17 The major alternative pathway is the binding of P-selectin glycoprotein ligand-1, a selectin-ligand expressed on T cells, to E and/or P selectin, which are induced on inflamed endothelium.Citation18,Citation19 The relative dominance of CD44 or selectins in regulating effector T-cell migration appears to be associated with environmental cues that include the magnitude and duration of inflammation.
In addition to the well-established role of mediating T-cell extravasation, CD44 also has a crucial function in regulating cell motility within the tissue stroma. In tissue-resident T cells, CD44 and other adhesion receptors accumulate at the trailing edge, or uropod, that is the part of the cell body that is in contact with the ECM.Citation20,Citation21 Interactions of ERM (ezrin/radixin/moesin) proteins with the cytoplasmic tail of CD44 can contribute to the stabilization of a polarized cell shape that is necessary for migration. ERM proteins regulate cytoskeletal organization through interactions with actinCitation22 and are involved in the generation of the uropod of a migrating T cell.Citation23 A recent study highlighted the importance of CD44-mediated polarity on interstitial migration in a cancer model, where anti-tumor activity of Cd44-/- CD8 T cells was compromised as a consequence of defective migration.Citation17 Thus, CD44 expression in T cells in the interstitial space may be essential to maintain cell shape during active migration. As movement takes place, CD44 anchorage of the cell's uropod to the ECM is disengaged by proteolytic activity. Cleavage of the extracellular domain (ECD) of CD44 is mediated by membrane type metalloproteinases (MT1-MMP) on responding T cells.Citation24 In tumor cells, metalloproteinases belonging to the ADAM (a disintegrin and metalloproteinase domain) family play an important role in ECD cleavage.Citation25 Unlike in T cells, CD44 localizes at the leading edge of the cell and is cleaved by ADAM-17, which allows for the redistribution of both molecules and movement of the cell. The resulting extension of the cell was proposed to upregulate Ca++-induced ADAM-10 activation in the uropod, where cleavage of CD44 and detachment from the ECM subsequently occur.Citation26 It is unknown if ADAM-10 and −17 have similar roles in T cells, especially since CD44 seems to be primarily located in the uropod.Citation20,Citation21 However, through its interaction with CD49d, CD44 may gain access to the leading edge of activated, migrating T cells where it would be able to interact with ADAM-17.Citation14
The release of soluble CD44 ECD could regulate the ability of T cells to interact with the ECM during migration by inhibition of CD44-dependent cell-cell and cell-matrix interactions.Citation27 Cleavage of CD44 ECD initiates the intra-membrane cleavage and release of the intracellular domain (ICD) by presenilin-1/γ secretase.Citation28,Citation29 The resulting cytoplasmic fragment translocates to the nucleus where it may be directly or indirectly involved in gene transcription.Citation30 At least one of the targets of the ICD is the transcriptional co-activator CBP/p300 and activation results in part in the upregulation of Cd44 itself and de novo CD44 production.Citation28 Whether this occurs in T cells is also unknown.
Thus, CD44 can function to mediate entry of T cells to target sites, as well as cell motility within these tissues. The rapid activation and proteolytic degradation of CD44 could allow efficient cell migration and as such is crucial for the potency of T cell effector functions.
Regulation of T-Cell Responses by CD44
In addition to regulating adhesion during T-cell migration, it is also apparent that CD44 has underappreciated roles in regulating effector T-cell responses. CD44 appears to be the predominant HA-binding protein normally expressed by activated T cells.Citation31 The binding of CD44 on the surface of T cells to HA on the surface of dendritic cells (DC) can promote cell clustering,Citation32 suggesting a role in T cell-DC conjugate formation, which is necessary for the induction of an immune response. In addition, CD44 is shown to be recruited to lipid rafts at the immunological synapse on DCs, where it contributes to the stability of DC-T cell interactions, as well as augmentation of T-cell proliferation and cytokine responses.Citation33 The ligand on T cells that promotes this effect has not been identified, but synthesis of HA by activated T cells has been described.Citation31
Although CD44 can directly regulate proliferation of various cell types,Citation34 and ligation has been reported to enhance T-cell proliferation to TCR signaling in vitro,Citation35,Citation36 we have not detected a direct role in modulating T-cell expansion during an immune response in vivo.Citation4 Although in vitro studies have suggested that CD44 can act as a co-stimulatory molecule for the production of cytokines from activated T cells, using adoptive transfer models, we have not found evidence for this function in vivo in studies of the immune responses of CD44-deficient CD4 T cells to influenza virus infection or antigen-presenting DCs.Citation4 Furthermore, CD44-deficient CD8 T cells do not have impaired induction of immune responses after adoptive transfer to normal recipients.Citation17 Nevertheless, we observe a function for CD44 in regulating the survival of CD4 T cells by sustaining the immune response, which is necessary for development of protective memory cells.Citation4 This effect was observed for Th1 CD4 cells, but not for other subsets of CD4 T cells or CD8 T cells. The death of CD4 T cells responding to influenza virus infection was recapitulated in CD44-deficient mice as well as by antibody-mediated blocking of HA-binding by CD44.
Additional studies have shown a non-redundant role of CD44 in the function of regulatory CD4 T cells (Tregs), which are essential for the prevention of autoimmunity. In vitro, Tregs from CD44-deficient mice have an impaired capacity to inhibit T-cell responses and diminished production of the cytokines, TGFα1 and IL-10,Citation37 which are necessary for their function. We find that CD44-deficient Tregs fail to persist after transfer in vivo (our unpublished observations). In vitro ligation of CD44 on activated wild-type Tregs promotes persistent expression of the transcription factor FoxP3, which is essential for regulatory activity.Citation37 These functions of CD44 are shown to depend upon interactions with high molecular weight forms of HA that are found in the absence of inflammatory responses.
Regulation of Activation-Induced Cell Death by CD44
Regulation of cell death is an important mechanism to maintain homeostasis of antigen-reactive T cells. The generation and expansion of effector T cells during a response requires survival signals, whereas the attrition of the cells after an infection depends upon regulated cell death. There is considerable evidence that CD44 can regulate apoptosis in T cells. However, roles in both resistance and susceptibility to cell death have been described, suggesting that it participates in the control of expansion. Some of the discrepancies are likely due to differences in the T cells and model systems that have been used, particularly antibody-mediated ligation of CD44. Both agonist and antagonist effects of CD44 can occur with induction or prevention of cell death in vitro, respectively.Citation38–Citation40 In vitro studies using T cells from CD44- deficient mice suggest that the absence of CD44 confers resistance to apoptosis and demonstrate that HA binding by wildtype cells elicits apoptosis.Citation39 In vivo, the absence of CD44 or the CD44 variant, v7, ameliorates T-cell dependent induced inflammation models that include arthritis and inflammatory bowel disease,Citation41,Citation42 but not others such as hepatitis.Citation5 Antibody-mediated blocking of CD44 in vivo generally has protective effects in models of chronic inflammatory disease.Citation5 Because CD44 is so broadly expressed on cells that are engaged in inflammatory responses, it is impossible to determine if generic CD44 blocking or deletion in T cells specifically ameliorated clinical signs.
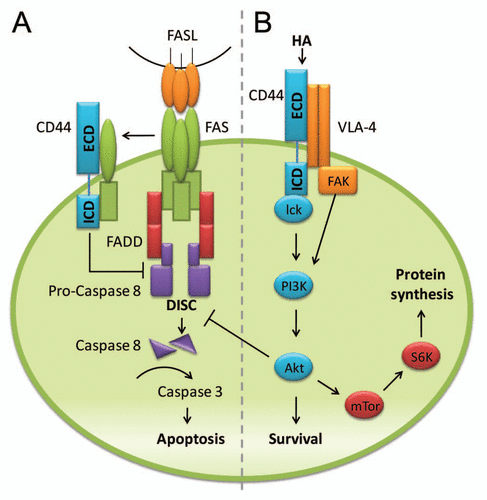
Several mechanisms by which CD44 can protect against cell death in T cells have been identified. CD44 has been shown to interact with the cytokine, osteopontin, which can provide a survival signal and protect from activation-induced cell death.Citation43 CD44 has been found to associate with death receptor, CD95 (FAS), on apoptosis-resistant tumor cells and has been proposed to inhibit apoptosis by sequestering FAS and preventing assembly of death-inducing signaling complex (DISC) ().
Our studies with the adoptive transfer model, where only CD4 T cells lacked CD44 expression, directly demonstrate a role in protection against cell death that is mediated by Fas engagement.Citation4 Our data further indicate that not all T cells are similarly regulated by CD44 since only Th1 cells are susceptible to death induced by the absence or blocking of CD44. In addition, Th1 cells express much higher levels of Fas than other T cells, including CD8 T cells and the Th2 and Th17 T cells. Our data also show that CD44 prevents caspase-8 activation in Th1 cells, implying interference with the DISC. Another mechanism by which CD44 can prevent apoptosis is by providing a survival signal, as discussed below.
Signal Transduction in T Cells via CD44
Ligation of CD44 can have an important role in signaling events that shape the immune response. However, CD44 lacks intrinsic signaling activity and the signaling pathways coupled to CD44 are not fully defined. Signaling can differ in different cell types and may depend on the proteins with which CD44 associates (e.g., VLA-4). Most studies have been performed with T-cell lines, and little is known regarding signaling events in primary T cells. CD44 engagement can facilitate aggregation of CD44-integrin-kinase signaling components in lipid rafts.Citation44,Citation45 Kinases that associate with the cytoplasmic tail of CD44 include the Src family kinases, Lck and Fyn,Citation44,Citation46,Citation47 which can be activated through HA binding. Src family kinase activity is necessary for CD44-induced actin rearrangement, which is required for cell spreading.Citation48 Lck appears to have a greater role than Fyn in CD44-mediated signaling in T cells and its function can be modulated by the transmembrane tyrosine phosphatase, CD45.Citation48 Ligation of CD44 by HA can also activate the PI3K/Akt signaling pathway, which is associated with cell survival in various cell types, including T cells.Citation40,Citation43 We have recently shown that CD44-induced Akt activation is particularly important for Th1 cells.Citation4 Activation of PI3K/Akt can inhibit Fas-mediated CD4 T-cell deathCitation49 by interfering with DISC assembly.Citation50 We hypothesize that this pathways is initiated by the binding of the intracellular domain of CD44 to Lck (). Both calcium influx and PI3K activation are thought to depend on Lck activation after CD44 ligation.Citation48,Citation51 However, PI3K could also be activated through FAK since CD44 co-localizes with VLA-4, which in turn binds FAK. Thus, ligation of either CD44 or VLA-4 could activate PI3K through FAK.Citation14,Citation15,Citation45,Citation52 Activation of the PI3K/Akt pathway is also associated with various aspects of T-cell migration, including actin reorganization and cell motility.Citation53 Thus, engagement of CD44 represents one means by which interactions with the environment and the ECM can be translated into a cellular response.
Pathways that mediate the effects of CD44 downstream of PI3K/Akt signaling have not been widely studied in T cells. However, we observe that PI3K/Akt activates p70 S6K downstream of the mammalian target of nutrient sensing rapamycin (mTOR) pathway (our unpublished results, ). mTOR, which can exist both in the TORC1 and TORC2 complexes, is activated both downstream and upstream of Akt. The TORC1 complex is essential for the differentiation of CD4 cells into Th1, Th2 and Th17 effector cells.Citation54 The mTOR pathway may be engaged during the effector T-cell response to support the survival and expansion through engagement of inhibitors of apoptosis that include the X-linked inhibitor of apoptosis protein (XIAP)Citation55 and survivin,Citation56 which target both intrinsic and extrinsic apoptosis cascades by inhibiting caspases. Recently, activation of mTOR in the TORC2 complex has been associated with the differentiation of Th1 and Th2 cells but not Th17 cells. Furthermore, TORC2 regulates Th1 cell differentiation via Akt, but Th2 cell differentiation through PKC.Citation57 Interestingly, TORC2 is necessary for the proliferation of T cells but not their survival.Citation57 Dissecting the role of CD44 signaling in primary T cells will provide more information on other functions of CD44 and may provide potential molecular targets that can be exploited to modulate T-cell responses during an ongoing immune response.
Conclusions
Although CD44 is associated with multiple functions in various cell types, it is now evident that this surface receptor has unique roles in regulating the survival of Th1 cells and the function of Tregs. This is surprising because CD44 is upregulated and maintained on all T cells that have been engaged in an immune response. However, Th1 cells may uniquely require additional survival signals through CD44 engagement of PI3K/Akt because of their elevated levels of Fas and inherent ability to rapidly assemble the DISC in response to Fas trimerization.Citation58 Such a mechanism may not be necessary in Th2 cells, and possibly other subsets of T cells, because of a greater capacity to engage PI3K/Akt in response to activation in addition to overall lower levels of Fas expression. Low molecular weight fragments of HA, which are generated during an immune response, may regulate Th1 cells, whereas Tregs may be maintained by high molecular weight HA to maintain tolerance to self tissues. It is likely that differences in the engagement of signaling pathways in the various T-cell subsets after ligation of CD44 account for their differing responses that impact the outcomes of effector and memory T-cell generation. CD44 has previously been shown to regulate cell migration, but it is now apparent that CD44 plays previously unappreciated key functions in T-cell responses to regulate immunity and control autoimmunity.
Figures and Tables
Figure 1 Potential mechanisms by which CD44 can modulate cell death. (A) CD44 may inhibit apoptosis by sequestering FAS and thereby preventing assembly of death-inducing signaling complex (DISC). Without DISC formation, Fas ligand (FasL) cannot engage FAS, which precludes downstream activation of caspases that would lead to apoptosis. (B) Ligation of CD44 (e.g., through HA) can facilitate aggregation of CD44-integrin-kinase signaling components in lipid rafts. Src family kinases, such as Lck, associate with the cytoplasmic tail of CD44 and activate the PI3K/Akt signaling pathway. Alternatively, binding of either CD44 or VLA-4, which form a heterodimer at the cell surface, could activate PI3K through FAK. Activation of PI3K/Akt is associated with cell survival. Akt can inhibit Fas-mediated CD4 T-cell death by interfering with DISC assembly. In addition, the mTOR pathway may be engaged to support the survival and expansion of T cells.
Acknowledgements
Mia Deiro and Dr Roberto Tinoco for critical evaluation of the manuscript. This work was supported by grants from NIH (AI061615 and AI046530) to L.M.B.
References
- Ponta H, Sherman L, Herrlich P. CD44: From adhesion molecules to signaling regulators. Nat Rev Molec Cell Biol 2003; 4:33 - 45
- Katoh S, Miyagi T, Taniguchi H, Matsubara Y, Kadota J, Tominaga A, et al. Cutting edge: an inducible sialidase regulates the hyaluronic acid binding ability of CD44-bearing human monocytes. J Immunol 1999; 162:5058 - 5061
- Skelton TP, Zeng C, Nocks A, Stamenkovic I. Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J Cell Biol 1998; 140:431 - 446
- Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 regulates survival and memory development in Th1 cells. Immunity 2010; 32:104 - 115
- Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets 2009; 8:208 - 220
- Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Molec Med 2001; 7:213 - 221
- Zoller M, McElwee KJ, Engel P, Hoffmann R. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol 2002; 118:983 - 992
- DeGrendele HC, Kosfiszer M, Estess P, Siegelman MH. CD44 activation and associated primary adhesion is inducible via T cell receptor stimulation. J Immunol 1997; 159:2549 - 2553
- Lesley J, Howes N, Perschl A, Hyman R. Hyaluronan binding function of CD44 is transiently activated on T cells druing an in vivo immune response. J Exp Med 1994; 180:383 - 387
- Ariel A, Lider O, Brill A, Cahalon L, Savion N, Varon D, et al. Induction of interactions between CD44 and hyaluronic acid by a short exposure of human T cells to diverse pro-inflammatory mediators. Immunology 2000; 100:345 - 351
- Levesque MC, Haynes BF. Activated T lymphocytes regulate hyaluronan binding to monocyte CD44 via production of IL-2 and IFNgamma. J Immunol 2001; 166:188 - 196
- Levesque MC, Haynes BF. Cytokine induction of the ability of human monocyte CD44 to bind hyaluronan is mediated primarily by TNFalpha and is inhibited by IL-4 and IL-13. J Immunol 1997; 159:6184 - 6194
- DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 1997; 278:672 - 675
- Marhaba R, Freyschmidt-Paul P, Zoller M. In vivo CD44-CD49d complex formation in autoimmune disease has consequences on T cell activation and apoptosis resistance. Eur J Immunol 2006; 36:3017 - 3032
- Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity 2004; 20:455 - 465
- Yago T, Shao B, Miner JJ, Yao L, Klopocki AG, Maeda K, et al. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood 2010; 116:485 - 494
- Mrass P, Kinjyo I, Ng LG, Reiner SL, Pure E, Weninger W. CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity 2008; 29:971 - 985
- Bonder CS, Clark SR, Norman MU, Johnson P, Kubes P. Use of CD44 by CD4+ Th1 and Th2 lymphocytes to roll and adhere. Blood 2006; 107:4798 - 4806
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol 2004; 4:325 - 335
- del Pozo MA, Sanchez-Mateos P, Nieto M, Sanchez-Madrid F. Chemokines regulate cellular polarization and adhesion receptor redistribution during lymphocyte interaction with endothelium and extracellular matrix. Involvement of cAMP signaling pathway. J Cell Biol 1995; 131:495 - 508
- Rosenman SJ, Ganji AA, Tedder TF, Gallatin WM. Syncapping of human T lymphocyte adhesion/activation molecules and their redistribution during interaction with endothelial cells. J Leukoc Biol 1993; 53:1 - 10
- Charrin S, Alcover A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front Biosci 2006; 11:1987 - 1997
- Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J 1999; 18:501 - 511
- Savinov AY, Rozanov DV, Golubkov VS, Wong FS, Strongin AY. Inhibition of membrane type-1 matrix metalloproteinase by cancer drugs interferes with the homing of diabetogenic T cells into the pancreas. J Biol Chem 2005; 280:27755 - 22758
- Nagano O, Murakami D, Hartmann D, De Strooper B, Saftig P, Iwatsubo T, et al. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol 2004; 165:893 - 902
- Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci 2004; 95:930 - 935
- Okamoto I, Kawano Y, Sasaki J, Nakao M, Matsumoto M, Suga M, et al. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene 1999; 18:1435 - 1446
- Okamoto I, Kawano Y, Murakami D, Sasayama T, Araki N, Miki T, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol 2001; 155:755 - 762
- Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, et al. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide. J Biol Chem 2002; 277:44754 - 44759
- Thorne RF, Legg JW, Isacke CM. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J Cell Sci 2004; 117:373 - 380
- Mummert ME, Mummert D, Edelbaum D, Hui F, Matsue H, Takashima A. Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J Immunol 2002; 169:4322 - 4331
- Do Y, Nagarkatti PS, Nagarkatti M. Role of CD44 and hyaluronic acid (HA) in activation of alloreactive and antigen-specific T cells by bone marrow-derived dendritic cells. J Immunother 2004; 27:1 - 12
- Hegde VL, Singh NP, Nagarkatti PS, Nagarkatti M. CD44 mobilization in allogeneic dendritic cell-T cell immunological synapse plays a key role in T cell activation. J Leukoc Biol 2008; 84:134 - 142
- Pure E, Assoian RK. Rheostatic signaling by CD44 and hyaluronan. Cell Signal 2009; 21:651 - 655
- Galandrini R, Galluzzo E, Albi N, Grossi CE, Velardi A. Hyaluronate is costimulatory for human T cell effector functions and binds to CD44 on activated T cells. J Immunol 1994; 153:21 - 31
- Sugiyama K, Komada Y, Deguchi T, Zhang XL, Azuma E, Ido M, et al. CD3-mediated T cell activation is inhibited by anti-CD44 monoclonal antibodies directed to the hyaluronan-binding region. Immunol Invest 1999; 28:185 - 200
- Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, et al. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10 and TGFbeta. J Immunol 2009; 183:2232 - 2241
- Nakano K, Saito K, Mine S, Matsushita S, Tanaka Y. Engagement of CD44 upregulates Fas Ligand expression on T cells leading to activation-induced cell death. Apoptosis 2007; 12:45 - 54
- Ruffell B, Johnson P. Hyaluronan induces cell death in activated T cells through CD44. J Immunol 2008; 181:7044 - 7054
- Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem 2001; 276:46024 - 46030
- Wittig BM, Johansson B, Zoller M, Schwarzler C, Gunthert U. Abrogation of experimental colitis correlates with increased apoptosis in mice deficient for CD44 variant exon 7 (CD44v7). J Exp Med 2000; 178:497 - 507
- Naor D, Nedvetski S. CD44 in rheumatoid arthritis. Arthritis Res Ther 2003; 5:105 - 115
- Larkin J, Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. CD44 differentially activates mouse NK T cells and conventional T cells. J Immunol 2006; 177:268 - 279
- Foger N, Marhaba R, Zoller M. CD44 supports T cell proliferation and apoptosis by apposition of protein kinases. Eur J Immunol 2000; 30:2888 - 2899
- Lee JL, Wang MJ, Sudhir PR, Chen JY. CD44 engagement promotes matrix-derived survival through the CD44-SRC-integrin axis in lipid rafts. Mol Cell Biol 2008; 28:5710 - 5723
- Rozsnyay Z. Signaling complex formation of CD44 with src-related kinases. Immunol Lett 1999; 68:101 - 108
- Taher TE, Smit L, Griffioen AW, Schilder-Tol EJ, Borst J, Pals ST. Signaling through CD44 is mediated by tyrosine kinases. Association with p56lck in T lymphocytes. J Biol Chem 1996; 271:2863 - 2867
- Wong NK, Lai JC, Birkenhead D, Shaw AS, Johnson P. CD45 downregulates Lck-mediated CD44 signaling and modulates actin rearrangement in T cells. J Immunol 2008; 181:7033 - 7043
- Varadhachary AS, Edidin M, Hanlon AM, Peter ME, Krammer PH, Salgame P. Phosphatidylinositol 3′-kinase blocks CD95 aggregation and caspase-8 cleavage at the death-inducing signaling complex by modulating lateral diffusion of CD95. J Immunol 2001; 166:6564 - 6569
- Jones RG, Elford AR, Parsons MJ, Wu L, Krawczyk CM, Yeh WC, et al. CD28-dependent activation of protein kinase B/Akt blocks Fas-mediated apoptosis by preventing death-inducing signaling complex assembly. J Exp Med 2002; 196:335 - 348
- Bourguignon LY, Lokeshwar VB, Chen X, Kerrick WG. Hyaluronic acid-induced lymphocyte signal transduction and HA receptor (GP85/CD44)-cytoskeleton interaction. J Immunol 1993; 151:6634 - 6644
- Su CC, Lin YP, Cheng YJ, Huang JY, Chuang WJ, Shan YS, et al. Phosphatidylinositol 3-kinase/Akt activation by integrin-tumor matrix interaction suppresses Fas-mediated apoptosis in T cells. J Immunol 2007; 179:4589 - 4597
- Ward SG, Marelli-Berg FM. Mechanisms of chemokine and antigen-dependent T-lymphocyte navigation. Biochem J 2009; 418:13 - 27
- Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology 2009; 127:459 - 465
- Zehntner SP, Bourbonniere L, Moore CS, Morris SJ, Methot D, St. Jean M, et al. X-linked inhibitor of apoptosis regulates T cell effector function. J Immunol 2007; 179:7553 - 7560
- Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity 2005; 22:621 - 631
- Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 2010; 32:743 - 753
- Varadhachary AS, Peter ME, Perdow SN, Krammer PH, Salgame P. Selective upregulation of phosphatidylinositol 3′-kinase activity in Th2 cells inhibits caspase-8 cleavage at the death-inducing complex: a mechanism for Th2 resistance from Fas-mediated apoptosis. J Immunol 1999; 163:4772 - 4779