Abstract
At 10 nM, ouabain elicits changes in cell contacts, which are independent and usually in opposite direction to effects occurring at μM levels, suggesting that these depend on entirely different mechanisms.1 However, this does not discard the possibility that in both instances ouabain would act on the same receptor. We demonstrate that such is the case by comparing the response of wild and ouabain-resistant MDCK cells on a very special type of cell contact, the tight junction (TJ).
The affinity and specificity of Na+,K+-ATPase for inhibitor ouabain are so high, that led to suspect that there might exist endogenous analogues. In keeping with this possibility Hamlyn et al.Citation2 demonstrated the presence in plasma of a substance that, so far, cannot be distinguished from ouabain by mass spectrometry.Citation2–Citation5 This endogenous ouabain (EO) is first and foremost an adrenocortical hormone that may be also synthesized and secreted by the hypothalamus.Citation6 EO was found to increase during exercise,Citation7 salty mealsCitation8 and pathological conditions such as arterial hypertensionCitation9 and myocardial infarctions,Citation10 raising the possibility that ouabain would constitute a new hormone (“new” in the sense that its status was only recently recognized), and prompting efforts to discover its physiological role.
In 1999 we observed that toxic levels of ouabain (≥1.0 µM) elicits retrieval from the plasma membrane of adhesion molecules to cell-cell and cell-substrate contacts between MDCK cells, and cause cell detachment (the so called “P→A mechanism”, from pump/adhesion).Citation11 On these basis we speculatedCitation1 that perhaps non-toxic levels of ouabain (≤50 nM) would act on molecules involved in cell adhesion, and enable hormone ouabain to modulate cell contacts. In keeping with this possibility we have recently found that 10 nM ouabain modifies the TJ through signaling pathways involving c-Src and ERK1/2, and distinctly modulating the expression as well as the pattern of distribution of specific claudins.Citation12 This selective modulation is also reflected by the fact that participation of these signal molecules on the regulation of flux of ions (as gauged through TER) is different from the modulation of the flux of neutral Dextran. Interestingly, these hormonal effects elicited by 10 nM ouabain, are just the opposite of toxic ones caused by levels 50 times higher of ouabain.Citation13 Therefore toxic and hormonal effects differ so much that leads to wonder whether they depend on different receptors.
Our basic strategy is to compare TER, a parameter that gauges the degree of tightness of the TJ, in the presence of inhibitors of signaling pathways, in wild (W-MDCK) and ouabain-resistant epithelial cells (R-MDCK). R cells constitute a stable line produced by Soderberg et al. by selection for growth in MDCK cells exposed to 2 µM ouabain, and have up to 30,000-fold higher Kd for this substance.Citation16 Other characteristics of Na+,K+-ATPase such as the rate of synthesis and amount of enzyme per unit of cell protein was unaltered in the mutants.Citation16 In the batch of MDCK cells that we use in our laboratory, W cells are totally detached from the monolayer by ouabain concentrations ≤1 µM in less than 15 hours; a similar effect on R cells would take concentrations two or three orders of magnitude higher.Citation17
Ouabain Increases TER in W-MDCK Cells But Not in R Ones
In circles correspond to W and squares to R-MDCK cells. Open and filled symbols indicate control and ouabain treated cells respectively. Untreated W and R cells have a similar degree of tightness of the TJ (TER at time zero). Yet 10 nM ouabain gradually increases TER in W cells (gray circles) but not in R ones (gray squares). The effect of ouabain on W cells is a slow one, reflecting the fact that hormonal effects depend on protein synthesis.Citation12 In the three day period studied the effect does not reach saturation. To study the possibility that R cells would be able to respond to ouabain albeit at higher concentrations, in we tested up to 1 µM, and corroborated that R cells do not respond to hormone ouabain. Notice that at this (toxic) concentration W cells do not respond by increasing the tightness of the TJ, but open this attaching structure so it becomes completely relaxed (black circles). Therefore, R cells, whose Na+,K+-ATPases cannot bind ouabain, are not stimulated by 10–100 nM of the hormone either, as if the pump were also the hormonal receptor.
c-Src (but Not IP3K) Participates in the Effect of Ouabain
Receptors are usually associated to molecules that convey information to diverse cell structures and mechanisms. In this respect the Na+,K+-ATPase associates to a variety signaling pathways, notably through c-Src, IP3K, PLC and ERK1/2.Citation18–Citation21 Therefore we explored the possibility that these molecules would also participate in the transmission of no toxic effects. This was tested by studying the sensitivity to specific inhibitors of c-Src, ERK ½; IP3K and EGFR in W- and R-MDCK cells. shows the TER across monolayers in control condition and stimulated by 10 nM ouabain (white and grey columns), a systematic control that accompanied every test in the present work. Third and fourth columns show the value of TER in the presence of 10 µM PP2, an inhibitor of c-Src (light blue) and 10 nM ouabain in the presence of the inhibitor (dark blue). Blocking c-Src prevents the response to ouabain. Fifth and sixth columns are represented in different shades of red, to illustrate that by itself, the inhibition of phosphoinositide 3-kinases (PI3K) with 25 µM LY294002 does not affect the value of TER (pink), and the stimulation of this parameter with 10 nM ouabain is not prevented when added simultaneously with the inhibitor (red). In summary: while the route of c-Src participates in the modulation of the TJ permeability to ions, iP3K does not.
With a similar protocol, in we blocked ERK1/2 with 10 µM PD98059 and observed that by itself it lowers the control value of TER in monolayers of W-MDCK cells (light green), but does not completely block the effect of ouabain on TER (dark green). The studies described in the previous paragraph show that ouabain stimulation of Na+,K+-ATPase activates c-Src, a molecule that can also transactivate the epidermal growth factor receptor (EGFR).Citation19 The EGFR autophosphorylation can be prevented with 10 µM PD153035 a specific inhibitor.Citation22 Yet the last two columns in (lila shades) show that the inhibitor by itself (lila) does not perturb TER, and when added in combination with 10 nM ouabain (dark lila) does not prevent the stimulating effect of the hormone. Therefore ERK1/2 (but not the autophosphorylation of EGFR) participates in the effect of hormone ouabain. Therefore, while ERK1/2 is one of the effectors of the pump, and participates in TJ modulation.
It should be taken into account that the purpose of the present studies is not to systematically explore all signaling routes involved in the effect of hormone ouabain, but further explore whether Na+,K+-ATPase may be the receptor of hormone ouabain, as in this case it should activate specific signaling routes and muscle fibers. The fact that this effect cannot be elicited in R-MDCK whose pumps have an extremely low affinity for ouabain due to a specific mutation ( and B) and ouabain does not engage signaling routes known to be associated to the pump ( and B), is in keeping with the possibility that the pump is the receptor of ouabain.
The physiological role of Na+,K+-ATPase is important in several respects: (1) It develops a Na+/K+ across the plasma membrane that gives rise to an electrical potential that is fundamental for the membrane electrical potential in all cells, in particular excitable ones like neurons a muscle fibers. (2) Na+,K+-ATPase is responsible for the low concentration of Na+ in the cytoplasm, that provides the driving force for most co- and countertransporters that operate the flux of nutrients (e.g., Na+/glucose, Na+/aminoacids, Na+/protons, Na+/Ca2+, Na+/K+, etc.) (3) It is also responsible for operating the exchange of substances across the whole transporting epithelium that makes metazoan life possible.
In the present article we provide evidence that Na+,K+-ATPase is also a receptor for the modulation that ouabain exerts on cell contacts. Ogawa et al.Citation23 have recently described a crystal structure of Na+,K+-ATPase from the shark rectal gland with ouabain bound by soaking the extract with 20 mM ouabain for 5 h. They found a single site for ouabain binding, with this molecule deeply inserted into the transmembrane domain with the lactone ring very close to the bound K+. Structural changes caused by ouabain binding are rather small and limited to transmembrane helices M1–M4, and are essentially the same for the high and the low-affinity ouabain-bound state. shows the contour of the crystallized Na+,K+-ATPase as well as the contour of ouabain (red) that we traced to ease discussion. Canessa et al.Citation24 have found that substituting a cysteine present in the wild type first transmembrane segment, by a tyrosine or a phenylalanine increases the inhibition constant of ouabain-resistant MDCK cells by more of three orders of magnitude. We marked the position of this mutation with a black circle () which is very close to the position occupied by ouabain. These findings rule out the possibility of two different sites, one for blocking the enzyme, and a second one for triggering hormonal effects. In R-MDCK the affinity of this site for ouabain is too low, and cannot act as hormonal receptor.
A couple of decades ago, cell contacts were regarded as mere mechanical structures that strengthen the tissues, lest these would disintegrate upon deformation. Today that there is considerable information on the large number and wide variety of protein species in tight, gap and adherent junctions, as well as synapses, the role of cell contacts can be hardly overestimated, as they are involved in differentiation, proliferation, communication, wound healing, cancer, metastases, etc. Suffice to say that the most complex object in the universe, the brain, assembles itself and its circuits on the basis of what cell touches what other, when and how.
In the last decade a growing body of information indicated that ouabain and a series of very closely related analogs are usually present in diverse tissues, as well as vary in physiological and pathological conditions, most probably constituting a hormone.Citation25 Following our demonstration that toxic levels of ouabain, besides blocking the pump, affect protein species participating in cell adhesion,Citation11 we proposed and offer experimental evidence that at non-toxic levels hormone ouabain may modulate cell contacts.Citation1,Citation12 The results of the present work favors the possibility that the receptor of hormone ouabain is the same Na+,K+-ATPase where most toxic effects are triggered. Na+,K+-ATPase modulates the hermeticity of the TJ through specific signaling paths such as c-Src and ERK1/2. However, it should be stressed that physiological effects are by no means comparable to attenuated toxic ones, as they usually vary in opposite directions.
Materials and Methods
Cell culture.
Starter MDCK-II cultures were obtained from the American Type Culture Collection (MDCK, CCL-34). Cells were maintained in DMEM supplemented (CDMEM) with 10% bovine serum and penicillin-streptomycin 10,000 U/mg/ml (In Vitro). Cells were harvested with trypsin-EDTA and plated on Transwell (∼2 × 105 cell/cm2, Corning Costar), maintained for tree days in CDMEM, reduce serum from 10 to 1% for 24 h, and then challenge the monolayer with ouabain. Inhibitors are added one hour before ouabain and renewed daily remaining present throughout.
Transepithelial electrical resistance (TER).
The degree of sealing of TJ to ions was assessed by measuring the TER of the cells grown on Transwell permeable supports using an EVOM (World Precision Instruments). TER was measured after 1–3 days of ouabain treatment. Final values were obtained by subtracting resistance of the bathing solution and an empty support, and the results are expressed as ohms per square centimeter (Ω/cm2).Citation14,Citation15
Figures and Tables
Figure 1 10 nM Ouabain increases TER in monolayers of W-MDCK but not R-MDCK. (A) TER as measured in monolayers incubated in the absence or presence of ouabain in MDCK W and MDCK-R for 3 days. (B) Effect on TER of several concentrations of ouabain on R-MDCK (squares) and 1.0 µM on W-MDCK cells (circles).
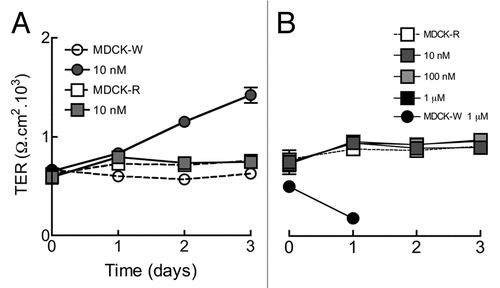
Figure 2 c-Src and ERK1/2 participate in the effect of ouabain on TER; EGFR autophosphorylation and IP3K do not. To ease comparisons, a given pair of related columns are represented in light and dark shades of the same color. (A) TER across monolayers of W-MDCK cells with or without ouabain, in the absence (white and gray columns) or presence of PP2 (light and dark blue columns) or LY294002 (pink and red columns). (B) TER across monolayers of W-MDCK cells with or without ouabain in the absence (white and gray columns) or presence of PD98059 (light and dark green columns) or PD153035 (lila and purple columns). Samples were taken at the 2nd day of adding ouabain.
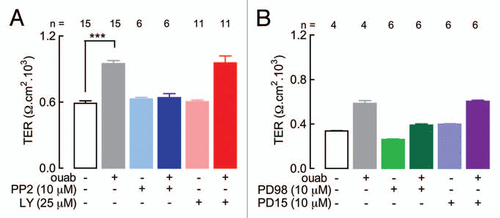
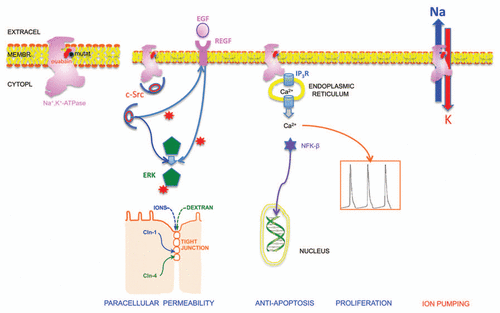
Figure 3 Relationship between Na+,K+-ATP ase (pink silhouette), the plasma membrane and several signaling routes and physiological effects (bottom). The silhouette corresponds to the contour of the crystal structure published by Ogawa et al. (2009). It shows also the position of ouabain (red), as well as the site where a cysteine residue in W cells was mutated in R ones, causing a marked decrease of their affinity for ouabain that allow them to survive in the presence of high concentrations of ouabain (black circle). In the second Na+,K+-ATPase (left to right) c-Src (red oval) is occluded and cannot phosphorylate EGF receptor nor ERK1/2 for example. Upon addition of ouabain, the c-Src is unmasked and is able to phosphorylate (red stars) both of them. c-Src and ERK1/2 cause a modification in the distribution of claudins 1 and 2 that changes the permeability of the TJ to ions and neutral Dextran. The third Na+,K+-ATPase illustrates the ability of ouabain to bind to this receptor and cause the release of Ca2+ from endoplasmic reticulum, that originates two basic responses; the first one is the transfer of NFKβ to the nucleus, where it modifies gene expression, and the second is the oscillation of calcium levels in the cytoplasm. These changes affect both apoptosis and proliferation. The Na+,K+-ATPase in the extreme right of the plasma membrane represents the well known toxic effect of treatment with µM levels of ouabain, which blocks this molecule as pump as well as ATP hydrolyzing enzyme. The consequence is of course an inhibition of Na+ and K+ pumping.
Acknowledgements
This work was supported by research grants and fellowships from the National Research Council of Mexico, of México City, and the Secretary of Health.
Addendum to:
References
- Larre I, Ponce A, Fiorentino R, Shoshani L, Contreras RG, Cereijido M. Contacts and cooperation between cells depend on the hormone ouabain. Proc Natl Acad Sci USA 2006; 103:10911 - 10916
- Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, et al. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci USA 1991; 88:6259 - 6263
- Kawamura A, Guo J, Itagaki Y, Bell C, Wang Y, Haupert GT Jr, et al. On the structure of endogenous ouabain. Proc Natl Acad Sci USA 1999; 96:6654 - 6659
- Komiyama Y, Nishimura N, Munakata M, Mori T, Okuda K, Nishino N, et al. Identification of endogenous ouabain in culture supernatant of PC12 cells. J Hypertens 2001; 19:229 - 236
- Schneider R, Wray V, Nimtz M, Lehmann WD, Kirch U, Antolovic R, et al. Bovine adrenals contain, in addition to ouabain, a second inhibitor of the sodium pump. J Biol Chem 1998; 273:784 - 792
- Blaustein JD. Progesterone and progestin receptors in the brain: the neglected ones. Endocrinology 2008; 149:2737 - 2738
- Bauer N, Müller-Ehmsen J, Krämer U, Hambarchian N, Zobel C, Schwinger RH, et al. Ouabain-like compound changes rapidly on physical exercise in humans and dogs: effects of beta-blockade and angiotensin-converting enzyme inhibition. Hypertension 2005; 45:1024 - 1028
- Manunta P, Iacoviello M, Forleo C, Messaggio E, Hamlyn JM, Lucarelli K, et al. High circulating levels of endogenous ouabain in the offspring of hypertensive and normotensive individuals. J Hypertens 2005; 23:1677 - 1681
- Manunta P, Hamilton J, Rogowski AC, Hamilton BP, Hamlyn JM. Chronic hypertension induced by ouabain but not digoxin in the rat: antihypertensive effect of digoxin and digitoxin. Hypertens Res 2000; 23:77 - 85
- Huang BS, Leenen FH. The brain renin-angiotensinaldosterone system: a major mechanism for sympathetic hyperactivity and left ventricular remodeling and dysfunction after myocardial infarction. Curr Heart Fail Rep 2009; 6:81 - 88
- Contreras RG, Shoshani L, Flores-Maldonado C, Lazaro A, Cereijido M. Relationship between Na+,K+-ATPase and cell attachment. J Cell Sci 1999; 112:4223 - 4232
- Larre I, Lazaro A, Contreras RG, Balda MS, Matter K, Flores-Maldonado C, et al. Ouabain modulates epithelial cell tight junction. Proc Natl Acad Sci USA 2010; 107:11387 - 11392
- Rajasekaran SA, Hu J, Gopal J, Gallemore R, Ryazantsev S, Bok D, et al. Na,K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am J Physiol Cell Physiol 2003; 284:1497 - 1507
- Cereijido M, Robbins ES, Dolan WJ, Rotunno CA, Sabatini DD. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol 1978; 77:853 - 880
- Flores-Benitez D, Rincon-Heredia R, Razgado LF, Larre I, Cereijido M, Contreras RG. Control of tight junctional sealing: roles of epidermal growth factor and prostaglandin E2. Am J Physiol Cell Physiol 2009; 297:611 - 620
- Soderberg K, Rossi B, Lazdunski M, Louvard D. Characterization of ouabain-resistant mutants of a canine kidney cell line, MDCK. J Biol Chem 1983; 258:12300 - 12307
- Bolívar JJ, Lázaro A, Fernández S, Stefani E, Peña-Cruz V, Lechene C, et al. Rescue of a wild-type MDCK cell by a ouabain-resistant mutant. Am J Physiol 1987; 253:151 - 161
- Mohammadi K, Kometiani P, Xie Z, Askari A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem 2001; 276:42050 - 42056
- Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem 2002; 277:18694 - 18702
- Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol 2007; 293:1489 - 1497
- Zhang L, Zhang Z, Guo H, Wang Y. Na+/K+-ATPase-mediated signal transduction and Na+/K+-ATPase regulation. Fundam Clin Pharmacol 2008; 22:615 - 621
- Bos M, Mendelsohn J, Kim YM, Albanell J, Fry DW, Baselga J. PD153035, a tyrosine kinase inhibitor, prevents epidermal growth factor receptor activation and inhibits growth of cancer cells in a receptor number-dependent manner. Clin Cancer Res 1997; 3:2099 - 2106
- Ogawa H, Shinoda T, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci USA 2009; 106:13742 - 13747
- Canessa CM, Horisberger JD, Louvard D, Rossier BC. Mutation of a cysteine in the first transmembrane segment of Na,K-ATPasealpha subunit confers ouabain resistance. EMBO J 1992; 11:1681 - 1687
- Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism and cell growth. Am J Physiol Cell Physiol 2007; 293:509 - 536