Abstract
The yeast dynamin-related GTPase Vps1 has been implicated in a range of cellular functions including vacuolar protein sorting, protein trafficking, organization of peroxisome, and endocytosis.1-2 Vps1 is present at endocytic sites and may be directly involved in endocytic vesicle invagination through its membrane-tubulating activity. Here, evidence supporting the functional link between Vps1 and the yeast amphiphysin Rvs167 in vesicle invagination is discussed. Though the disassembly of endocytic factors from pinched-off endocytic vesicles appears to be tightly regulated in a spatiotemporal manner, we are far from having complete understanding of the underlying mechanism. In this study, we provide evidence that Vps1 plays a role in the uncoating of endocytic proteins from post-internalized vesicles, based on the observation of a quick disassembly of two endocytic coat proteins Ent1 and Ent2 in cells lacking Vps1.
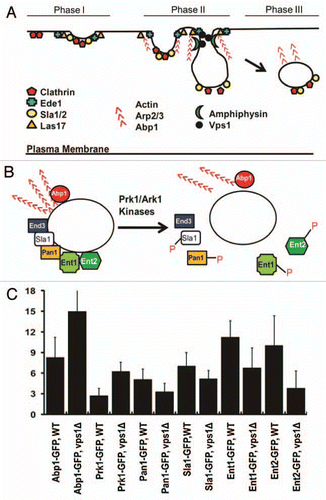
Endocytosis is a process by which extracellular materials and components of the plasma membrane are internalized. Internalized materials are then either targeted to the lysosome (vacuole) for degradation or recycled back to the plasma membrane to maintain membrane homeostasis.Citation3,Citation4 The hallmark events of endocytic internalization include invagination of the plasma membrane, followed by the pinching off of the invaginated endocytic vesicle.Citation3–Citation6 The temporal order of recruitment of endocytic components to endocytic sites in budding yeast has been considerably well documented (). In Phase I, early endocytic patch factors, including clathrin, Ede1, Las17 and Sla1/2, are recruited to a selected endocytic site.Citation7–Citation9 Phase II begins with recruitment of actin and its associated proteins (Arp2/3 and Abp1), and is coupled to membrane invagination, which is mediated by actin polymerization.Citation7 The membrane invagination then deepens and eventually detaches from the plasma membrane to form an endocytic vesicle by a process known as scission. Yeast amphiphysins (Rvs161/167) capable of tabulation and fragmentation of membranes are recruited to endocytic sites right after the actin machinery assembled in phase II, facilitating the scission ().Citation10 In Phase III, endocytic patches move away from the membrane, displaying a rapid and directional motility. The rapid burst of actin assembly mediated by the Arp2/3 complex pushes the patches farther away from the membrane.Citation8,Citation11
A recent fluorescence microscopic study of yeast dynamin Vps1 provided the first direct evidence that Vps1 arrives at the cortical endocytic sites around the same time as Rvs167.Citation2 This raises the interesting question on the biochemical targeting mechanism of Vps1 to endocytic sites. One possibility is that Vps1 might be recruited to endocytic sites by the early endocytic adaptor Sla1, given the fact that they biochemically interacts with each other.Citation12 Considering that Sla1 has also been known to bind directly to Rvs167,Citation13 it is tempting to speculate Sla1 being responsible for recruiting both Vps1 and Rvs167. Another possibility is that Vps1 is directly recruited to the target site via its ability to interact with the plasma membrane, based on the finding that self-assembled Vps1 oligomers are able to bind to and tubulate liposomes in vitro.Citation2 Nonetheless, the membrane tubulation ability of Vps1 appears surprising, because Vps1 contains neither the lipid binding Pleckstrin homology (PH) domainCitation14,Citation15 nor the proline-rich repeat domain required for self-assembly of classic dynamin.Citation16,Citation17 In light of findings that the I649K mutation in the C-terminal GED (GTPase Effector Domain) of Vps1 abolished Vps1's abilities to self-assemble and to tubulate liposomes, and that the liposome tubulation was accomplished without the activity of the N-terminal GTPase domain of Vps1,Citation2 it is plausible to conclude that Vps1's functions in membrane-binding and tubulating are attributed to the C-terminal GED of the protein. The physiological relevance of the Vps1's membrane-tubulating activity on endocytosis was uncovered by the same group of researchers using an ultrastructural EM study.Citation2 The authors showed that loss of Vps1 led to structural aberrations of invaginated endocytic pits with a significant increase in the angle of invagination. More importantly, most of the invaginations in the vps1 mutant cells were shallow rather than pronounced or deepened, indicating that the membrane-tubulating activity of Vps1 is required for further deepening of endocytic invagination. In view of the fact that yeast amphiphysin complex (Rvs161-Rvs167) binds to liposomes and promotes tubule formation,Citation18 one can postulate that Vps1 may work in concert with amphiphysins in order to increase efficiency in endocytic scission, as has been suggested from the studies in mammalian cells.Citation19,Citation20 Indeed, codeletion of Rvs167, but not of Rvs161, in the vps1Δ background caused a synthetic lethal phenotype at the non-permissive temperature (37°C) (our unpublished data, Smaczynska-de et al., 2010), suggesting a redundant role for Vps1 in modulating endocytic scission, namely endocytic invagination and its subsequent scission. Notably, our double vps1Δrvs167Δ mutant displayed not only a markedly increased lifespan of Abp1-GFP at endocytic sites but also a severe failure (>85%) in endocytic scission (unpublished data), the latter of which is consistent with the finding of a previous report in reference Citation2. Besidesits membrane-tubulating activity, it has been shown that Rvs167 forms multiple interaction with other endocytic proteins important for endocytic internalization, suggesting that it may function as a scaffold in endocytosis.Citation21 Considering that mammal amphiphysin has been proposed to function as a linker between the clathrin coat and dynamin in the endocytosis of synaptic vesicle,Citation22,Citation23 the further studies of physical connection between Rvs167 and Vps1 and the physiological relevance of their interaction are of great interest.
Though the spatiotemporal recruitment of endocytic factors to endocytic sites has now been analyzed in detail, relatively little is known about the mechanism behind the disassembly of endocytic coat and adaptor proteins as well as actin binding proteins from the endocytosed vesicles. A protein kinase family (Prk1 and Ark1) has been found to play a central role in the disassembly of endocytic coat and adaptor proteins, including Sla1, Ent1/2 and Pan1.Citation24–Citation26 The homologous protein kinases, Prk1 and Ark1, may function in phosphorylating their target endocytic proteins (), and the phosphorylated proteins dissociate from the endocytic vesicles to be reused for the next round of endocytosis. Previously, we reported that Vps1 deficient cells exhibit a significantly extended cortical lifespan of Abp1-GFP.Citation1 In this study, we further analyzed the effect of Vps1 loss on the disassembly of endocytic components. After detached from the cell cortex, patches initiate uncoating of endocytic proteins. The mean time required for uncoating of Abp1-GFP in the cytoplasm was ∼8 s for WT cells, much shorter compared to ∼15 s for vps1Δ cells (). We next sought to measure the uncoating time of Prk1-GFP. As shown in , the cytoplasmic uncoating time of Prk1-GFP in vps1Δ cells (6.3 ± 1.3 s) was much longer than that in WT cell (2.7 ± 1.1 s), and this difference was statistically significant (p < 0.01). We next assessed the effect of the slow uncoating of Prk1 on the disassembly of its known substrates. Interestingly, Ent1-GFP and Ent2-GFP were found to dissociate rapidly in the vps1 mutant as shown in , and there were statistically significant differences in the mean uncoating times of Ent1-GFP and Ent2-GFP between WT and vps1Δ cells (p < 0.01). The mean uncoating times of Pan1-GFP and Sla1-GFP in the mutant cells were slightly decreased, but the differences were not statistically significant (p > 0.01) (). It is likely that the kinase activity of Prk1 towards its substrates such as Ent1 and Ent2 remains active during the increased lifetime of Prk1 (∼4 more s) on the post-internalized endocytic patches in the vps1 mutant cells. Our speculation is that the delay of Prk1 uncoating may mimic in part the effect of hyperactivation of Prk1, followed by a relatively quick release of those adaptor proteins. Our results thus support in essence the notion that Prk1 is essential for disassembly of the coat and actin module proteins. Taken together, to our knowledge, our results provide the first evidence that the phosphorylation activity of Prk1 specifically promotes the disassembly of the two Epsin-like proteins Ent1 and Ent2 from the post-internalized vesicles.
According to a previous report in reference Citation25, the disassembly of Pan1 in Prk1-overexpressed cells was not altered. It is important to note that our seemingly conflicting results regarding the dynamics of Pan1 is mainly due to the fact that Zeng and coworkers (2001) reported the effect of Pan1 phosphorylation on its targeting to or dissociation from the cortical endocytic sites upon overexpressing Prk1, rather than monitoring its dissociation from the post-internalized vesicles. It thus may be that the phosphorylation of Pan1 by Prk1 is sufficient for disrupting the cortical Sla1-End3-Pan1 complex, but does not serve as a signal for its release from the complex before endocytic scission.
A recent live cell imaging analysis reported more disassembly factors, including Sjl2, Arf3, Gts1 and Lsb5.Citation28 The authors proposed that Sjl2, a PIP2 phosphatase, play a role in the disassembly of endocytic proteins such as Ent1, Ent2 and Sla2, based on the observation that those endocytic coat proteins persist on the post-internalized compartments upon loss of Sjl2. Given that the levels of membrane PIP2 in the absence of Sjl2 increase significantly (our unpublished data) and that those coat proteins contain a PIP2-binding domain,Citation28 it is likely that local reduction of PIP2 in the membrane via the Sjl2 phosphatase activity might be essential for effi- cient disassembly of the coat proteins. They showed that loss of Arf3, Gts1 or Lsb5 led to subtle defects in the disassembly of Sla1, but the mechanism underlying Arf3, Gts1 and Lsb5-mediated disassembly of Sla1 is not clear, awaiting further studies.
Figures and Tables
Figure 1 The spatiotemporal regulation of the assembly and disassembly of endocytic factors. (A) Recruitment of endocytic machinery to the cortical endocytic sites. Note that a yeast dynamin-like protein Vps1 and the amphiphysins are recruited after initiation of actin assembly. (B) Uncoating of endocytic factors. The phosphorylation activity of the homologous protein kinases, Prk1 and Ark1, has been implicated in the disassembly of endocytic proteins, including Sla1, Pan1, Ent1 and Ent2. (C) The mean uncoating times for GFP-fused endocytic factors. The time spent by a post-internalized vesicle carrying GFP-fused protein in the cytoplasm was determined.
Acknowledgements
This work was supported by a National Scientific Foundation Grant 0923024 (to K. Kim) and by thesis funding from Missouri State University (to D. Wang).
Addendum to:
References
- Nannapaneni S, Wang D, Jain S, Schroeder B, Highfill C, Reustle L, et al. The yeast dynamin-like protein Vps1:vps1 mutations perturb the internalization and the motility of endocytic vesicles and endosomes via disorganization of the actin cytoskeleton. Eur J Cell Biol 2010; 89:499 - 508
- Smaczynska-de Rooi II, Allwood EG, Aghamohammadzadeh S, Hettema EH, Goldberg MW, Ayscough KR. A role for the dynamin-like protein Vps1 during endocytosis in yeast. J Cell Sci 2010; 123:3496 - 3506
- Shaw JD, Cummings KB, Huyer G, Michaelis S, Wendland B. Yeast as a model system for studying endocytosis. Exp Cell Res 2001; 271:1 - 9
- Liu J, Sun Y, Drubin DG, Oster GF. The mechanochemistry of endocytosis. PLoS Biol 2009; 7:1000204
- Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature 2006; 439:998 - 1003
- Drubin DG, Kaksonen M, Toret C, Sun Y. Cytoskeletal networks and pathways involved in endocytosis. Novartis Found Symp 2005; 269:35 - 42
- Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors and actin during endocytic internalization. Cell 2003; 115:475 - 487
- Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 2005; 123:305 - 320
- Newpher TM, Smith RP, Lemmon V, Lemmon SK. In vivo dynamics of clathrin and its adaptordependent recruitment to the actin-based endocytic machinery in yeast. Dev Cell 2005; 9:87 - 98
- Dawson JC, Legg JA, Machesky LM. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol 2006; 16:493 - 498
- Kim K, Galletta BJ, Schmidt KO, Chang FS, Blumer KJ, Cooper JA. Actin-based motility during endocytosis in budding yeast. Mol Biol Cell 2006; 17:1354 - 1363
- Yu X, Cai M. The yeast dynamin-related GTPase Vps1p functions in the organization of the actin cytoskeleton via interaction with Sla1p. J Cell Sci 2004; 117:3839 - 3853
- Stamenova SD, Dunn R, Adler AS, Hicke L. The Rsp5 ubiquitin ligase binds to and ubiquitinates members of the yeast CIN85-endophilin complex, Sla1-Rvs167. J Biol Chem 2004; 279:16017 - 16025
- Shin HW, Takatsu H, Mukai H, Munekata E, Murakami K, Nakayama K. Intermolecular and interdomain interactions of a dynamin-related GTPbinding protein, Dnm1p/Vps1p-like protein. J Biol Chem 1999; 274:2780 - 2785
- Wang DS, Shaw G. The association of the C-terminal region of beta I sigma II spectrin to brain membranes is mediated by a PH domain, does not require membrane proteins, and coincides with a inositol-1,4,5-triphosphate binding site. Biochem Biophys Res Commun 1995; 217:608 - 615
- Hinshaw JE, Schmid SL. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature 1995; 374:190 - 192
- Warnock DE, Baba T, Schmid SL. Ubiquitously expressed dynamin-II has a higher intrinsic GTPase activity and a greater propensity for self-assembly than neuronal dynamin-I. Mol Biol Cell 1997; 8:2553 - 2562
- Youn JY, Friesen H, Kishimoto T, Henne WM, Kurat CF, Ye W, et al. Dissecting BAR domain function in the yeast Amphiphysins Rvs161 and Rvs167 during endocytosis. Mol Biol Cell 2010; 21:3054 - 3069
- Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 2009; 17:811 - 822
- Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell 2005; 9:791 - 804
- Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol 2003; 19:287 - 332
- McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, et al. A presynaptic inositol- 5-phosphatase. Nature 1996; 379:353 - 357
- David C, McPherson PS, Mundigl O, de Camilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci USA 1996; 93:331 - 335
- Watson HA, Cope MJ, Groen AC, Drubin DG, Wendland B. In vivo role for actin-regulating kinases in endocytosis and yeast epsin phosphorylation. Mol Biol Cell 2001; 12:3668 - 3679
- Zeng G, Yu X, Cai M. Regulation of yeast actin cytoskeleton- regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p. Mol Biol Cell 2001; 12:3759 - 3772
- Zeng G, Cai M. Regulation of the actin cytoskeleton organization in yeast by a novel serine/threonine kinase Prk1p. J Cell Biol 1999; 144:71 - 82
- Fazi B, Cope MJ, Douangamath A, Ferracuti S, Schirwitz K, Zucconi A, et al. Unusual binding properties of the SH3 domain of the yeast actin-binding protein Abp1: structural and functional analysis. J Biol Chem 2002; 277:5290 - 5298
- Toret CP, Lee L, Sekiya-Kawasaki M, Drubin DG. Multiple pathways regulate endocytic coat disassembly in Saccharomyces cerevisiae for optimal downstream trafficking. Traffic 2008; 9:848 - 859