Abstract
The mechanisms responsible for the oncogenic effects of the hyaluronan (HA) receptor and mitotic spindle binding protein, RHAMM, are poorly understood. On one hand, extracellular RHAMM interacts with HA and cell-surface receptors such as CD44 to coordinately activate the MAPK/ERK1,2 pathway, thus contributing to the spread and proliferation of tumor cells. On the other hand, intracellular RHAMM decorates mitotic spindles and is necessary for spindle formation and progression through G2/M and overexpression or loss of RHAMM can result in multi-pole spindles and chromosome mis-segregation. The deregulation of these intracellular functions could lead to genomic instability and fuel tumor progression. This suggests that both extracellular and intracellular RHAMM can promote tumor progression. Intracellular RHAMM can bind directly to ERK1 to form complexes with ERK2, MEK1 and ERK1,2 substrates, and we present a model whereby RHAMM’s function is as a scaffold protein, controlling activation and targeting of ERK1,2 to specific substrates.
RHAMM/HMMR: Hyaluronic Acid Receptor and Tumor-Associated Antigen
The hyaluronan receptor RHAMM/HMMR (also known as CD168) has attracted attention recently because its intracellular and extracellular matrix functions appear to play a role in both tumor susceptibility and progression. RHAMM is generally not detected in homeostatic tissues, but is transiently produced during wound repair and is constitutively expressed in many cancer types including carcinomas of the breast, prostate, gastrointestinal track, as well as aggressive forms of multiple myeloma, leukemias and lymphomas.Citation1–Citation11 This expression profile has led to the proposal that RHAMM is a promising target for cancer therapy. Currently, the exploration of the biology of RHAMM in tumor progression has led to novel cancer therapies that target the extracellular functions of RHAMM. Pre-clinical, phase I and II trials are currently underway targeting RHAMM as a novel tumor associated antigen (TAA) using a RHAMM-derived peptide vaccine to promote immune recognition and destruction of tumors by activated T cells.Citation4,Citation10,Citation11 Furthermore, RHAMM has been identified as a promising target for antibody therapy to block the extracellular function of RHAMM on the surface of tumor cells.Citation5 These approaches rely on the presence of RHAMM on the cell-surface but disregard the potential oncogenic roles of RHAMM inside cells. The mechanisms by which extracellular or intracellular RHAMM actually promote tumorigenesis remain poorly understood but it is essential to clarify them in order to develop therapeutic approaches that effectively block the oncogenic functions of this gene product.
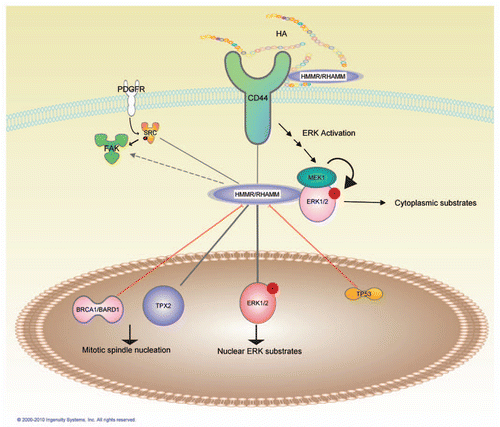
At the cell surface, extracellular RHAMM, which is not an integral membrane protein, regulates hyaluronan (HA) and growth factor-induced MAPK (ERK1,2) signaling via associations with transmembrane HA receptors such as CD44,Citation12 and PTK receptors such as PDGFR and RON ().Citation13 This signaling function of extracellular RHAMM is required for tumor cell motility and invasion.Citation12,Citation14 By contrast, intracellular RHAMM localizes to several subcellular compartments, including elements of the cytoskeleton and nucleus where it performs a variety of functions in addition to its role as an HA receptor. One function that has particularly captured the interest of cancer biologists is its involvement in Ran-dependent mitotic spindle formation. In vitro analyses suggest that RHAMM promotes mitotic spindle pole formation in response to Ran gradients, a function that is antagonized by BRCA1/BARD1 complexes.Citation15 The same study also showed that RHAMM protein levels are important in determining this effect on spindle formation. For example, when soluble recombinant RHAMM is added in excess to cells depleted of BRCA1/BARD1 complexes, it results in multi-pole spindles and improper aster formation. Furthermore, these defects are rescued when RHAMM-specific function blocking antibodies are added to the BRCA1/BARD1 depleted cells. Conversely, the absence of RHAMM protein in the presence of BRCA1/BARD1 complexes resulted in similar spindle and aster defects. The results of these experiments are further supported by evidence that micro-injected anti-RHAMM antibodies also result in multi-pole spindlesCitation16 and our recent report showing that genetic loss of RHAMM promotes aberrant mitotic spindles and mis-segregation of chromosomes.Citation17 This suggests that efficient Ran-dependent spindle formation requires an optimal concentration of intracellular RHAMM that is partially regulated by the BRCA1/BARD1 complex.
Collectively the above observations promoted interest in RHAMM as a novel breast cancer susceptibility gene with the potential to promote genomic instability. They did not however elucidate the mechanisms for these RHAMM-dependent mitotic spindle functions. We recently published evidence that intracellular RHAMM, like the cell surface form, regulates sustained activation of MEK1/ERK1,2. We also showed that purified recombinant RHAMM protein can bind to monomeric tubulin, and we hypothesize that intracellular RHAMM may link these MAP kinases to a pool of tubulin that is dynamically remodeling. Loss of RHAMM protein results in increased microtubule stability, the formation of multi-pole spindles and abnormal chromosome segregation.Citation17 Dynamic instability of microtubules and spindle formation can be rescued by low expression of either RHAMM or mutant active MEK1. This suggests a model in which RHAMM/tubulin interactions may affect mitotic spindle integrity by contributing to MEK1/ERK1,2 mediated dynamic instability of specific pools of tubulin. Importantly, similarities in the functions of intracellular and extracellular RHAMM raise the possibility that both pools of RHAMM are part of a larger sophisticated and novel network of integrated inside/outside signaling within the context of MAPK signaling.
Is RHAMM a Scaffold Protein?
Intracellular RHAMM protein levels are tightly regulated during cell cycle progression by means of ubiquitination by the anaphase promoting complex (APC).Citation18 This appears to be important for regulating the interaction of RHAMM with other spindle assembly factors such as TPX2.Citation16,Citation19 This leads to discrete temporal control of spindle formation during mitosis and provides the correct levels of RHAMM for proper spindle formation. The physical association of RHAMM with both ERK1 and tubulin, the ability of mutant active MEK1 to rescue spindle abnormalities in RHAMM−/− cells, and the manner by which RHAMM protein levels are tightly controlled are all consistent with the hypothesis that RHAMM functions as a scaffold protein which targets MEK1/ERK1,2 complexes to their substrates, in particular microtubules, whose stability (or lack thereof) impacts on signaling, motility and cell cycle.
ERK1,2 scaffolds are well recognized as important for targeting ERK to diverse cellular locations. In astrocytes, PEA-15,Citation20 functions as a scaffold to link nuclear ERK to RSK2, and the F-actin binding protein calponin appears to act as a scaffold to target ERK to SmAV at the cell membrane.Citation21 Within the context of the mitotic spindle, RHAMM may link active ERK to microtubule-associated substrates. In the absence of RHAMM, (either germline deleted or antibody-inhibited), ERK activity becomes inefficiently targeted and spindle formation is compromised. When RHAMM is in excess, RHAMM may sequester substrates and ERK away from each other leading to inefficient and aberrant ERK targeting. This is consistent with work suggesting that scaffold proteins require an optimum concentration to promote close association between an activator and their respective targets.Citation22
Our work thus illuminates a novel link between the ability of RHAMM to activate the MAPK pathway both from the cell surface and within the cell. On the cell surface, RHAMM may act as an HA scaffold, promoting signaling through CD44, while intracellular RHAMM acts as a MAPK/ERK scaffold to facilitate regulated activation of cell proliferation and cell migration. The extracellular and intracellular scaffolding functions of RHAMM may work in concert to co-ordinate cell migration and proliferation in response to the microenvironment. Given the importance of RHAMM in mitotic spindle formation and chromosome segregation, it is surprising that RHAMM deficient mice are not only viable but also not prone to the development of spontaneous tumors. It may be that RHAMM function is only required during specific pathologies associated with inflammation, wound repair, or cancer where a quickly changing microenvironment requires constant adjustment of cell behavior. A more thorough understanding of RHAMM's role in regulating dynamic crosstalk between cells and their microenvironment may open up new avenues for development of more targeted therapies for conditions associated with chronic inflammation, or for reducing the morbidity and mortality associated with malignant tumors.
Figures and Tables
Figure 1 RHAMM/HMMR-mediated MAPK signaling and interactions. RHAMM can interact directly with ERK1, CD44 and TPX2 and RHAMM expression is co-regulated with p53 and FAK. RHAMM may act as an adapter protein targeting activated ERK to various cellular locations including the mitotic spindle and cytoskeleton. This figure was generated through the use of Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com).
Addendum to:
References
- Crainie M, Belch AR, Mant MJ, Pilarski LM. Overexpression of the receptor for hyaluronan-mediated motility (RHAMM) characterizes the malignant clone in multiple myeloma: Identification of three distinct RHAMM variants. Blood 1999; 93:1684 - 1696
- Giannopoulos K, Dmoszynska A, Kowal M, Rolinski J, Gostick E, Price DA, et al. Peptide vaccination elicits leukemia-associated antigen-specific cytotoxic CD8+ T-cell responses in patients with chronic lymphocytic leukemia. Leukemia 2006; 24:798 - 805
- Greiner J, Ringhoffer M, Taniguchi M, Schmitt A, Kirchner D, Krähn G, et al. Receptor for hyaluronan acid-mediated motility (RHAMM) is a new immunogenic leukemia-associated antigen in acute and chronic myeloid leukemia. Exp Hematol 2002; 30:1029 - 1035
- Greiner J, Schmitt A, Giannopoulos K, Rojewski MT, Götz M, Funk I, et al. High dose RHAMM-R3 peptide vaccination for patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and multiple myeloma (MM). Haematologica 2010; 95:1191 - 1197
- Gust KM, Hofer MD, Perner SR, Kim R, Chinnaiyan AM, Varambally S, et al. RHAMM (CD168) is overexpressed at the protein level and may constitute an immunogenic antigen in advanced prostate cancer disease. Neoplasia 2009; 11:956 - 963
- Kalmyrzaev B, Pharoah PD, Easton DF, Ponder BA, Dunning AM. Hyaluronan-mediated motility receptor gene single nucleotide polymorphisms and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2008; 17:3618 - 3620
- Maxwell CA, Keats JJ, Belch AR, Pilarski LM, Reiman T. Receptor for hyaluronan-mediated motility correlates with centrosome abnormalities in multiple myeloma and maintains mitotic integrity. Cancer Res 2005; 65:850 - 860
- Maxwell CA, McCarthy J, Turley E. Cell-surface and mitotic-spindle RHAMM: Moonlighting or dual oncogenic functions?. J Cell Sci 2008; 121:925 - 932
- Nagel S, Hirschmann P, Dirnhofer S, Gunthert U, Tzankov A. Coexpression of CD44 variant isoforms and receptor for hyaluronic acid-mediated motility (RHAMM, CD168) is an international prognostic index and C-MYC gene status-independent predictor of poor outcome in diffuse large B-cell lymphomas. Exp Hematol 2010; 38:38 - 45
- Schmitt M, Schmitt A, Rojewski MT, Chen J, Giannopoulos K, Fei F, et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma elicits immunologic and clinical responses. Blood 2008; 111:1357 - 1365
- Tabarkiewicz J, Giannopoulos K. Definition of a target for immunotherapy and results of the first peptide vaccination study in chronic lymphocytic leukemia. Transplant Proc 2010; 42:3293 - 3296
- Hamilton SR, Fard SF, Paiwand FF, Tolg C, Veiseh M, Wang C, et al. The hyaluronan receptors CD44 and rhamm (CD168) form complexes with ERK1,2 that sustain high basal motility in breast cancer cells. J Biol Chem 2007; 282:16667 - 16680
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem 2002; 277:4589 - 4592
- Tolg C, Hamilton SR, Nakrieko KA, Kooshesh F, Walton P, McCarthy JB, et al. Rhamm−/− fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J Cell Biol 2006; 175:1017 - 1028
- Joukov V, Groen AC, Prokhorova T, Gerson R, White E, Rodriguez A, et al. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell 2006; 127:539 - 552
- Groen AC, Cameron LA, Coughlin M, Miyamoto DT, Mitchison TJ, Ohi R. XRHAMM functions in ran-dependent microtubule nucleation and pole formation during anastral spindle assembly. Curr Biol 2004; 14:1801 - 1811
- Tolg C, Hamilton SR, Morningstar L, Zhang J, Zhang S, Esguerra KV, et al. RHAMM promotes interphase microtubule instability and mitotic spindle integrity through MEK1/ERK1/2 activity. J Biol Chem 2010; 285:26461 - 26474
- Song L, Rape M. Regulated degradation of spindle assembly factors by the anaphase-promoting complex. Mol Cell 2010; 38:369 - 382
- Maxwell CA, Keats JJ, Crainie M, Sun X, Yen T, Shibuya E, et al. RHAMM is a centrosomal protein that interacts with dynein and maintains spindle pole stability. Mol Biol Cell 2003; 14:2262 - 2276
- Vaidyanathan H, Opoku-Ansah J, Pastorino S, Renganathan H, Matter ML, Ramos JW. ERK MAP kinase is targeted to RSK2 by the phosphoprotein PEA-15. Proc Natl Acad Sci USA 2007; 104:19837 - 19842
- Appel S, Allen PG, Vetterkind S, Jin JP, Morgan KG. h3/Acidic calponin: An actin-binding protein that controls extracellular signal-regulated kinase1/2 activity in nonmuscle cells. Mol Biol Cell 2010; 21:1409 - 1422
- Levchenko A, Bruck J, Sternberg PW. Scaffol proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc Natl Acad Sci USA 2000; 97:5818 - 5823