Abstract
The filamentous (F)-actin regulatory protein cortactin plays an important role in tumor cell movement and invasion by promoting and stabilizing actin related protein (Arp)2/3-mediated actin networks necessary for plasma membrane protrusion. Cortactin is a substrate for ERK1/2 and Src family kinases, with previous in vitro findings demonstrating ERK1/2 phosphorylation of cortactin as a positive and Src phosphorylation as a negative regulatory event in promoting Arp2/3 activation through neuronal Wiskott Aldrich Syndrome protein (N-WASp). Evidence for this regulatory cortactin “switch” in cells has been hampered due to the lack of phosphorylation-specific antibodies that recognize ERK1/2-phosphorylated cortactin. Our findings with phosphorylation-specific antibodies against these ERK1/2 sites (pS405 and pS418) indicate that cortactin can be co-phosphorylated at 405/418 and tyrosine residues targeted by Src family tyrosine kinases. These results indicate that the ERK/Src cortactin switch is not the sole mechanism by which ERK1/2 and tyrosine phosphorylation events regulate cortactin function in cell systems.
Motility-based processes in normal and transformed cells are governed by signal transduction pathways that regulate actin cytoskeletal dynamics. Actin regulatory proteins that serve as substrates downstream of multiple kinase cascades are important intersection points in integrating and controlling motile and invasive activities. Cortactin is an F-actin binding adaptor protein initially identified as a Src substrate in v-Src transformed cells.Citation1,Citation2 Subsequent work identified three tyrosine residues within the cortactin proline-rich carboxyl-terminal domain (Y421, Y470 and Y486 in humans) that are phosphorylated by Src and other tyrosine kinases.Citation3,Citation4 Analysis of these tyrosine residues using phenylalanine point mutants indicates the importance of their phosphorylation in cell motility and tumor cell metastasis.Citation3,Citation5 Tyrosine phosphorylated cortactin localizes within lamellipodia of motile cells and invadopodia in invasive carcinoma cells,Citation6,Citation7 supporting a functional role in cell movement and invasion.
Cortactin is a substrate for multiple serine/threonine kinases in addition to serving as a tyrosine kinase substrate.Citation8 ERK1/2 is a prominent serine/threonine kinase that phosphorylates cortactin at serine 405 and 418 in response to epidermal growth factor receptor (EGFR) activation.Citation9 Cortactin contains a Src homology (SH)3 domain at its carboxyl terminal end that is capable of binding and activating N-WASp, resulting in enhanced Arp2/3 actin nucleation activity.Citation10,Citation11 In 2004, Martinez-Quiles et al. examined the functional impact of ERK1/2 and Src phosphorylation on the ability of cortactin to regulate N-WASp activity. This report utilized purified protein components in in vitro actin polymerization assays to demonstrate that ERK1/2 phosphorylation substantially enhances the ability of cortactin to bind and activate N-WASp, promoting Arp2/3 actin nucleation. In contrast, Src phosphorylation of cortactin prevents cortactin binding to N-WASp and ablates the ability of phosphomimetic 405/418 cortactin mutants to promote N-WASp activation. These results led to the proposal of an on-off switch mechanism for cortactin regulation of N-WASp activity, whereby ERK1/2 cortactin phosphorylation liberates the cortactin SH3 domain from an undefined autoinhibitory state (presumed to be a SH3 domain binding site within the proline-rich domain), allowing it to bind and activate N-WASp. Src phosphorylation terminates this activity, enabling cortactin to return to its inactive confirmation. Based on this model, ERK1/2 phosphorylation and Src tyrosine phosphorylation are functionally interdependent events.
Subsequent studies utilizing exogenously expressed phosphomimetic and phosphorylation incompetent S405/418 mutants supports a role for ERK1/2 cortactin phosphorylation in promoting intracellular actin polymerizationCitation12 and actin-dependent invadopodia matrix degradation activity.Citation13 Our development of site-specific antibodies against cortactin pS405 and pS418 allows for the first direct cellular evaluation of these phosphorylation sites. We demonstrate that pS418 cortactin localizes to lamellipodia and invadopodia in carcinoma cells, supporting the identified role for cortactin in actin polymerization derived from studies with point mutant constructs. Significantly, these antibodies, along with commercially available antibodies against cortactin pY421 (and likely anti-pY470/pY486 or equivalent antibodies), provide a means for directly assessing cortactin serine and tyrosine phosphorylation status derived from cellular preparations. Cortactin is phosphorylated on S405/418 and Y421 in lysates from EGF-stimulated UMSCC2 head and neck squamous carcinoma cells, indicating that ERK1/2 and Src (or other cortactin-targeting tyrosine kinases) are activated and phosphorylate cortactin following EGFR activation. Analysis of phosphorylation-null point mutants with anti-pY421 and pS405 antibodies indicates that ERK1/2 and cortactin tyrosine phosphorylation are not interdependent events, since the inability of cortactin to become tyrosine phosphorylated does not prevent ERK1/2 phosphorylation (and vice versa). Furthermore, cortactin immunoprecipitated with anti-pS405 antibodies is phosphorylated on pY421 (), demonstrating co-phosphorylation of these sites on the same cortactin molecule. These data indicate that at least a subpopulation of cortactin within tumor cells is simultaneously phosphorylated by ERK1/2 and tyrosine kinases.
Our findings provide evidence that the ERK/Src cortactin switch is not the primary phospho-regulatory cortactin mechanism employed by cells. Recent studies indicate that cortactin tyrosine phosphorylation promotes actin polymerization through recruitment of the adaptor NCK1, which in turn binds and activates N-WASp and Arp2/3 to stimulate actin network formation in invadopodia.Citation7,Citation14,Citation15 The concurrent ability of cortactin to activate N-WASp by both tyrosine and ERK1 phosphorylation events allows for amplification of Arp2/3-mediated actin polymerization based on specific signaling input, providing a means for fine-tuning actin regulation at dynamic membrane structures during migration and invasion. Cortactin regulation of actin networks is likely more complex, given the ability of the cortactin SH3 domain to interact and activate several proteins in addition to N-WASp that signal to control Arp2/3 activation or actin dynamics.Citation16–Citation20 The presence of tyrosineCitation21 and serine phosphorylated cortactin in head and neck squamous cell carcinoma tumors suggests that both tyrosine- and serine-based signaling is relevant in neoplastic progression.
In addition to the apparent redundant roles of tyrosine and serine phosphorylation in N-WASp activation, these cortactin phosphorylation events can also have divergent cellular functions in migration. In two-dimensional systems, cortactin tyrosine phosphorylation alters focal adhesion turnover, whereas serine 405/418 phosphorylation stimulates actin polymerization and motility.Citation12 Our work extends these findings by demonstrating that serine 405/418 phosphorylation is required for dominant lamellipodia persistence, whereas tyrosine phosphorylation has no effect on lamellipodia dynamics (Ammer and Weed, unpublished data). This suggests that there are context-specific roles for cortactin tyrosine and serine phosphorylation in tumor cell motility () and is in agreement with the ability of these phosphorylation events to regulate different aspects of endocytic membrane trafficking.Citation22–Citation24 Continued deciphering of the complex pathways that impinge on and emanate from serine- and tyrosine-phosphorylated cortactin continues to present interesting and challenging avenues for understanding how these signals are utilized and integrated during different phases of cancer cell motility.
Figures and Tables
Figure 1 Cortactin is co-phosphorylated on S405 and Y421. OSC19 oral squamous carcinoma cells serum starved for 24 h were stimulated with 100 ng/ml EGF for 20 min as indicated. Cell lysates were immunoprecipitated with anti-cortactin pS405 antibodies and analyzed by western blotting with anti-cortactin pY421 and pan-cortactin antibodies. Total cell lysates were evaluated for the presence of pS405 cortactin, pY421 cortactin, total cortactin and β-actin.
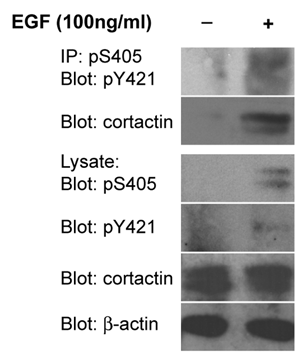
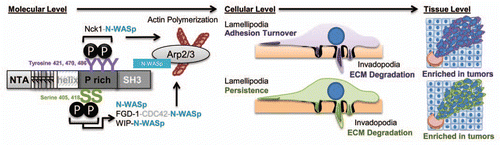
Figure 2 Mechanistic and cellular roles of cortactin tyrosine and serine phosphorylation in tumor cell motility. Tyrosine (purple) and serine (green) phosphorylation events are shown depicting direct and indirect pathways that ultimately result in N-WASp activation. The effects of cortactin serine and tyrosine phosphorylation result in common net outcomes in invadopodia function (ECM degradation), but differ in impact on lamellipodia dynamics as shown. Cortactin phosphorylated on serine and tyrosine residues is enriched in human tumors, where it likely enhances invasive and metastatic capacity.
Acknowledgements
This work was supported by a subproject of NIH grant P20-RR16440 to S.A.W.
Addendum To:
References
- Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol 1991; 11:5113 - 5124
- Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol 1993; 120:1417 - 1426
- Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J Biol Chem 1998; 273:25770 - 25776
- Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskel 2008; 65:687 - 707
- Li Y, Tondravi M, Liu J, Smith E, Haudenschild CC, Kaczmarek M, et al. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res 2001; 61:6906 - 6911
- Head JA, Jiang D, Li M, Zorn LJ, Schaefer EM, Parsons JT, et al. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell 2003; 14:3216 - 3229
- Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, et al. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci 2010; 123:3662 - 3673
- Martin KH, Jeffery ED, Grigera PR, Shabanowitz J, Hunt DF, Parsons JT. Cortactin phosphorylation sites mapped by mass spectrometry. J Cell Sci 2006; 119:2851 - 2853
- Campbell DH, Sutherland RL, Daly RJ. Signaling pathways and structural domains required for phosphorylation of EMS1/cortactin. Cancer Res 1999; 59:5376 - 5385
- Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res 2002; 62:669 - 674
- Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol 2004; 24:5269 - 5280
- Kruchten AE, Krueger EW, Wang Y, McNiven MA. Distinct phospho-forms of cortactin differentially regulate actin polymerization and focal adhesions. Am J Physiol Cell Physiol 2008; 295:1113 - 1122
- Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tetí S, Luini A, et al. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci 2008; 121:369 - 378
- Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci USA 2007; 104:11933 - 11938
- Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol 2009; 186:571 - 587
- Kinley AW, Weed SA, Weaver AM, Karginov AV, Bissonette E, Cooper JA, et al. Cortactin interacts with WIP in regulating Arp2/3 activation and membrane protrusion. Curr Biol 2003; 13:384 - 393
- Hou P, Estrada L, Kinley AW, Parsons JT, Vojtek AB, Gorski JL. Fgd1, the Cdc42 GEF responsible for Faciogenital Dysplasia, directly interacts with cortactin and mAbp1 to modulate cell shape. Hum Mol Genet 2003; 12:1981 - 1993
- Schafer DA, Weed SA, Binns D, Karginov AV, Parsons JT, Cooper JA. Dynamin2 and cortactin regulate actin assembly and filament organization. Curr Biol 2002; 12:1852 - 1857
- Dudek SM, Birukov KG, Zhan X, Garcia JG. Novel interaction of cortactin with endothelial cell myosin light chain kinase. Biochem Biophys Res Commun 2002; 298:511 - 519
- Le Clainche C, Pauly BS, Zhang CX, Engqvist-Goldstein AE, Cunningham K, Drubin DG. A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. EMBO J 2007; 26:1199 - 1210
- Ammer AG, Kelley LC, Hayes KE, Evans JV, Lopez-Skinner LA, Martin KH, et al. Saracatinib impairs head and neck squamous cell carcinoma invasion by disrupting invadopodia function. J Cancer Sci Ther 2009; 1:52 - 61
- Cao H, Chen J, Krueger EW, McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the “constitutive” endocytosis of transferrin. Mol Cell Biol 2010; 30:781 - 792
- Zhu J, Yu D, Zeng XC, Zhou K, Zhan X. Receptor-mediated endocytosis involves tyrosine phosphorylation of cortactin. J Biol Chem 2007; 282:16086 - 16094
- Grassart A, Meas-Yedid V, Dufour A, Olivo-Marin JC, Dautry-Varsat A, Sauvonnet N. Pak1 phosphorylation enhances cortactin-N-WASP interaction in clathrin-caveolin-independent endocytosis. Traffic 2010; 11:1079 - 1091