Abstract
Positioning of centrosomes within cells determines the directionality of cell division, as well as directionality of cellular activities in the interphase. This brief review focuses on similarities (and differences) of centrosome positioning during early divisions in the Caenorhabditis embryo and during the interaction of T lymphocytes with other cells in the course of immune response. In the study of the two phenomena, a synergy of experimentation and numerical mechanical analysis has recently been achieved. The picture that emerges from these studies is one in which simple physical forces under the constraints of the basic cell structure lead to complex, "life-like" mechanical behavior. This behavior includes instability of equilibria, irreversibility of structural transitions, and multidimensional, multiperiodic oscillations. This new picture of cell mechanics may form an interesting paradigm for future research.
CentrosomesCitation1 serve as microtubule-organizing centers in interphase animal cells and as spindle poles during mitosis. As such, their positioning in the cell confers directionality to cellular activities such as secretion and division, and is important in both development and the adult organism. Considerable attention has been given recently to the biomechanical underpinnings of centrosome positioning in two widely different systems: the spindle orientation in worm embryos and the centrosome polarization in T lymphocytes. Despite or perhaps owing to their wide evolutionary and functional separation, the two systems exhibit instructive similarities and the computational and experimental research that has been done in the two areas is proving to be complementary. The comparative biomechanics of the two systems is the subject of this brief review. For a discussion of the spindle orientation and lymphocyte polarization in their respective broader contexts, and from the signaling and genetic perspective, the reader may be referred to excellent recent reviews in reference Citation2 and Citation3.
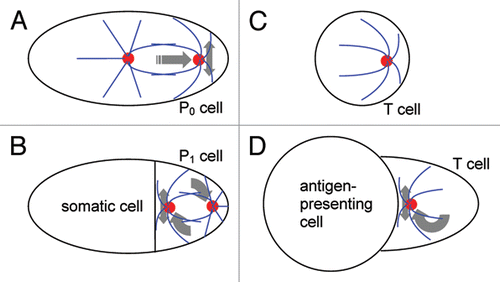
Mitotic spindle positioning during the first divisions in Caenorhabditis elegansCitation4 is but one example from the wide variety of casesCitation2 where the positioning of spindle poles is responsible for directional growth and cell differentiation. Before the first division of this worm embryo, the spindle is oriented lengthwise in the elongated cell, which is called the P0 cell. The spindle also moves closer to that end of the cell which corresponds to the future posterior end of the embryo. Transverse oscillationsCitation5 of the leading spindle pole accompany this movement (). The division that follows is asymmetric: the proximity of the spindle to the future posterior pole results in unequal partitioning of the mother cell cytoplasm. At the two-cell stage, the anterior cell is relatively large, and the posterior cell is small. The posterior cell also exclusively inherits the fate determinants that make it part of the germ line,Citation6,Citation7 whereas the anterior cell is the first somatic cell of the new organism. The posterior cell (P1) then undergoes a second division, which is aligned with the same anterior-posterior axis of the embryo. The posterior daughter cell resulting from this second division (the P2 cell) continues the germ line, while the more anterior daughter becomes another somatic cell. The alignment of the second division axis with the anterior-posterior axis of the embryo is once again responsible for the unequal partitioning of the cell fate determinants. Yet the two poles of the second spindle are initially located on the transverse axis. One of them must move to the interface of the P1 cell with the anterior cell to align the spindle properlyCitation4 (). Moving on an arc, this centrosome often “overshoots,” and oscillations about the middle position follow.Citation8
T lymphocytes perform their functions in the mammalian immune system by directly interacting with other cells.Citation3 In suspension, these white blood cells are essentially spherical and display an eccentric position of the centrosomeCitation9 (). Upon conjugation with a cell that presents on its surface a foreign or abnormal molecular fragment (antigen), the T cell positions its centrosome next to the cell-cell interface (). The importance of this position is demonstrated particularly vividly in the experimentCitation10 where a T cell conjugates simultaneously with multiple B cells, which are a class of “professional” antigen-presenting cells in the immune system. Despite all the B cells presenting the same antigen in this experiment, only the one toward which the T-cell centrosome is oriented becomes stimulated and starts dividing to enhance the immune response. This is due to the association with the centrosome of the secretory vesicles that contain the immunological effector and mediator molecules; the proximity of the centrosome to a specific site of the T-cell surface makes their secretion narrowly directed.Citation9,Citation11–Citation13 The T-cell centrosome reorienting to the site of contact with the antigen-presenting cell may “overshoot” and display oscillations around the middle of the contact areaCitation14 ().
Analogies between centrosome positioning in worm embryos and T lymphocytes stand out in juxtaposition (). The single-cell stage in the worm and the isolated T cell both display a capacity for autonomous polarization ( and C). The posterior (P1) cell at the two-cell stage in Caenorhabditis, and the T cell conjugated with an antigen-presenting cell both display polarization with respect to the cell-cell contact ( and D). Analogy between centrosome positioning in various cell types appears to be the rule rather than exception.Citation2,Citation15 Juxtaposition of the T- and P-cell cases, however, presents a special interest, because in addition to biochemical and genetic, they have been subject to biomechanical investigation.
Mechanics of Oscillations
The oscillations are perhaps the most striking feature of centrosome positioning in the two systems. The reasoning that they must be revealing of the nature of the forces that drive centrosome positioning in general was behind the effort to decipher their mechanics. It was established by means of numerical analysis and experimental testsCitation16–Citation18 that two types of forces—pushing and pulling—can, when combined, generate the spindle pole oscillations in the single-cell worm embryo (). Both forces are mediated by microtubules that radiate from the centrosome and extend to the cell boundary. In many cell types it is found that pulling force can be exerted by the cell boundary, or by specialized areas of the cell cortex, on microtubules that contactit.Citation15,Citation19–Citation21 This force can be generated by molecular motors, especially those of the dynein type or by capture of microtubule ends which then depolymerize, generating force. The pulling force is opposed by pulling on the opposite side of the cell, and by bending and pushing of microtubules against the cell boundary on the same side. In the middle, the centrosome should, according to intuition, be in equilibrium, as the microtubules on all sides should then be equally pushing against the boundary and equally pulled by the boundary. Yet the calculationsCitation16–Citation18 show that oscillations of the centrosome position around the middle will arise spontaneously in this system. This emergent behavior in the mechanical model is counterintuitive, but it is life-like and explains the oscillations seen at the single-cell stage in Caenorhabditis.
The T cells that conjugate with antigen-presenting cells () display a tight apposition of the centrosome with the flattened cell-cell interface. They also have comparatively long microtubules. As a result, the centrosome undergoing the oscillations effectively slides along the inner surface of the cell-cell contact zone on microtubules that are bent against this surface.Citation14 Kim and MalyCitation22 simulated the bending of the microtubules and the pulling force exerted on them by dynein that accumulates on the inner surface of the interface.Citation23,Citation24 Similarly to the numerical models of the Caenorhabditis elegans zygote, they found that the equilibrium does not persist, and oscillations develop spontaneously. Notably, their model did not include any statistical “noise” that mimicked the molecular-level stochasticity in the worm models. This shows that the oscillations are an intrinsic feature of the deterministic mechanics of centrosome positioning. Beyond simple oscillations, the centrosome in the T cell model was describing a three-dimensional trajectory, in which multiple periods and amplitudes along each dimension could be discerned. Overall, the comparison of the computed movement with the experimental videos obtained by Kuhn and PoenieCitation14 demonstrates how life-like a movement can be that is driven by only a few types of simple physical forces in the cell's structural framework.
The simulations with long, strongly bent microtubules in T cells revealed a new effect, whose role seemed to be dominant in the three-dimensional oscillations: the microtubules on the trailing side of the centrosome are lifted off the pulling surface by viscous drag in the cytoplasm.Citation22 Thus, the pulling on the leading side always wins, no matter which direction the centrosome is moving to—until the movement is ended by bending of the leading microtubules against the cell boundary. The microtubules that trailed then have time to relax elastically and come in contact with the pulling surface again to initiate the movement to the opposite side. While these observations may serve to explain the oscillatory cycle, they cannot intuitively explain why the oscillations do not die down or why the system does not remain in equilibrium with the centrosome in the middle. As an emergent behavior of the mechanical system which is the cell, the oscillations can only be rigorously, albeit non-intuitively, explained through the precise numerical analysis of the biomechanical model. The particularly close structural similarity between the conjugated T cell and the P1 cell of Caenorhabditis elegans ( and D) suggests that the mechanism of oscillations in the P1 cell may involve the same microtubule sliding and lift-off effect as in the T cell.
Mechanics of Autonomous Asymmetry
The displacement of the spindle from the center of the P0 cell in Caenorhabditis elegans toward the posterior pole () is driven by the asymmetric distribution of force-generating elements, such as dynein, that pull on astral microtubules.Citation21,Citation25,Citation26 The concentration of the pulling on the posterior cortex ensures that the spindle is shifted toward the posterior granules, which determines the fate of the smaller daughter cell as a germ-line cell.Citation6,Citation7 In contrast, the isolated T cell in suspension () has no frame of reference in which its polarity must develop. The eccentric positioning of the centrosome in this caseCitation9 corresponds simply to the energy minimumCitation27–Citation29 of the microtubules, which are anchored at the centrosome and bend against the cell boundary. Calculations show that the elastic energy of bending is not minimized when all microtubules are bent equally. Rather, a conformation in which some microtubules are bent more, and the other ones are bent less, corresponds to the minimum of the bending energy of the entire cytoskeleton. Thus, the autonomous polarity of the centrosome position is explained in the same way as molecular conformations are explained—by energy minimization—albeit with counterintuitive results.
HolyCitation30 was the first to calculate (in two dimensions) that the energy minimum of a microtubule cytoskeleton confined inside the cell corresponds to an asymmetric conformation and eccentric position of the centrosome. Earlier, the pioneering quantitative mechanical theory of spindle positioning by BjerknesCitation31 predicted that microtubule buckling and pushing against the cell surface should result in positions that would be equidistant from the cell surface. The equality of the microtubule pushing force in the centered position indeed establishes the condition of mechanical equilibrium. It does not, however, by itself establish the condition of stability of this equilibrium. Unstable equilibria do not persist, and therefore are usually not seen in nature, as the case of a ball on top of a ball will illustrate. At the same time, the lowest-energy state is not necessarily adopted by the system, as illustrated by a ball sitting on a table, while its lower-energy position would be on the floor. An equilibrium is rendered stable if a deviation from it causes a restoring force in the system. A displacement of a centrosome to the right from the equidistant equilibrium position will cause a stronger bending of the microtubules to the right of it, and it will relax the microtubules that push against the cell surface on the left. From these intuitive considerations, it appears that the net effect should be a force that would restore the equidistant equilibrium position. However, Maly and MalyCitation29 conducted a rigorous numerical stability analysis and showed that the centered position of the centrosome is unstable. Deviations from symmetry actually cause a force that only further increases the deviation, until a new, eccentric equilibrium is reached. This holds in three dimensions as well as in two (in typical flat cultured cells), but only in three dimensions, like in the voluminous T cells, does the eccentric equilibrium indeed correspond to the minimum of energy.Citation29 Thus, the mechanics of confined cytoskeletons is more complex not only in comparison with intuitive expectations, but also in comparison with numerical predictions that are based exclusively on calculating the static equilibrium or energy minimum.
What has already been elucidated about the mechanics of single-cell Caenorhabditis embryos and isolated T cells appears to correlate well with the differences in the biological function that is served by the asymmetry in the two cases. In P0 cells, the asymmetry must correspond with the asymmetry of the distribution of cell fate determinants, and this end is served well by the asymmetric pulling on the microtubule cytoskeleton. In isolated T cells, an arbitrary eccentric position produced by the spontaneous symmetry-breaking would seem to suffice functionally, because the specific orientation only becomes important after the conjugation with an antigen-presenting cell. However, given the structural similarities between the two systems, the following questions should be worth investigating. Can the spindle in P0 cells acquire its position through spontaneous symmetry-breaking if, by analogy with the T-cell cytoskeleton, it is energetically advantageous that microtubules emanating from one spindle pole bend more strongly against the cell boundary than do the microtubules of the other pole? The asymmetry of pulling could then bias this spontaneous symmetry-breaking in the direction which is correct in the developmental sense. Similarly, can the microtubule cytoskeleton in the suspended T cells be pulled on asymmetrically toward a certain, as yet undiscovered, cortical site? Such a site, while being arbitrarily oriented in the laboratory frame of reference, could nonetheless provide a directional bias for the thermodynamic tendency of the cytoskeleton toward arbitrary eccentricity. If the study of biochemical systems is any guide to the new field of systems biomechanics, it should not indeed be surprising if the two systems possessed an additional level of complexity beyond what appears “sufficient” from the functional viewpoint, and if there existed a redundancy arising from conservation of the entire mechanical repertoire.
Attraction to the Cell Contact
Both T cells and P1 cells position centrosomes in close apposition to the area of their contact with the other cell: an antigen-presenting cell in the immunological interaction and the somatic cell in the two-cell embryo ( and D). Notably, the cell contact area is flattened in both cases—in fact, even concave—thus differing from the rest of the T or P1 cell surface, which is convex. In both T and P1 cells, the molecular motor dynein or its adapter dynactin was found in the cortex underlying the contact patch.Citation23,Citation32 Observations and experiments also indicated that pulling forces are transmitted by microtubules from the contact area to the centrosome in P1 and T cells.Citation14,Citation33 This is consistent with the cortically anchored dynein pulling on microtubules that contact the patch, and thus “reeling in” the centrosome. (In P1 cells, one of the two centrosomes, chosen at random, is pulled anteriorly, while the connection through the disintegrating nucleus and forming spindle pushes the opposite spindle pole posteriorly,Citation4 like at seesaw.) The involvement of dynein and dynactin into the positioning of the centrosome to the contact was further supported by molecular genetic experiments on P1 and T cells.Citation24,Citation26,Citation32
The results of Tsou et al.Citation34 demonstrate that in P1 cells, the effects of cell shape and polarized pulling are largely redundant. This follows from experiments with mutating the par-3 gene and directly manipulating the shape of the cell. PAR-3 is a higher-level regulator of cell polarity that is capable of initiating assembly of a protein complex that involves dynein.Citation2 It is localized to the anterior cortex of P1 cells at the cell-cell interface.Citation35 When a wild-type P1 cell is made round in the experiment, the centrosome rotation proceeds normally. Similarly, it proceeds normally if the molecular polarity is disrupted in par-3 mutants, but the cell's shape is left unmanipulated. That it is the cell shape that drives the movement in the mutants is demonstrated by reorientation of the centrosome to an ectopic flattened area that can be created experimentally. To abolish the centrosome orientation in P1 cells, it is necessary to both mutate par-3 and make the cell round.Citation34 These experiments indicate that the cell makes use of two widely different mechanisms to build up redundancy behind a centrosome movement that is critical to the organism's development.
In view of the close structural and dynamical analogy between the two systems, numerical modeling of T cells appears to provide explanation also for the experiments on P1 cells. It was found by means of computational analysis that the dynein pulling in T cells can indeed “reel in” the centrosome to the cell-cell interface, even considering the significant biophysical constraints such as the molecular motor force, microtubule rigidity, and viscous resistance of the cytoplasm.Citation22 At the same time, the cited work demonstrated that the pulling mechanism of positioning is error-prone, and in fact unlikely to reorient the microtubule cytoskeleton by more than 90 degrees. One of the reasons for this stands out particularly clearly in the complexity of the cytoskeleton movements that are induced by the localized cortical pulling in the mechanical model. Long-range reorientation requires long microtubules that would stretch from the centrosome and come in contact with the cortical patch where dynein is concentrated. However, such long microtubules are susceptible to “jamming” in the confined cytoplasmic space, and thus not allowing the reorientation to complete. For certain initial conditions (which in reality are unique for each cell), the “jamming” may become spontaneously resolved through a catastrophic stability loss of the static equilibrium in the “jammed” cell.Citation22 This is not unlike the loss of stability that can lead to the emergence of cell-autonomous polarity. Still, a large fraction of cells attempting long-range reorientation of the centrosome are predicted not to be able to complete it.
The prediction that pulling by itself should be unreliable in T cells led to experimental discovery of mechanisms that act before and after pulling to make it less error-prone and to actively correct its errors. One of these auxiliary mechanisms reorients the entire T cell by a significant angle in the very beginning of the cell-cell interaction.Citation36 This first-stage reorientation reduces the angle through which the pulling mechanism needs to reorient the centrosome to achieve the fully functional orientation. The other mechanism accelerates the disengagement from the antigen-presenting cell of those T cells that cannot achieve the functional polarity.Citation37,Citation38 This appears to make the entire composite process “fail-safe.” Obviously, neither reorientation of the entire P1 cell, nor disengagement from the somatic cell is an option in the worm embryo. In P1 cells, however, a centrosome is never more than 90 degrees away from its final functional position, since there are two diametrically opposed centrosomes to choose from.Citation4 From the viewpoint afforded by the mechanical model of the T cell, therefore, the initial conditions in P1 cells are comparatively conducive to successful reorientation by pulling.
The cell shape effects also find explanation in numerical models that were developed for T cells. It was established by means of computational analysis that positioning of the centrosome next to a flattened patch on the otherwise convex cell surface minimizes energy.Citation27,Citation28 The energy in these calculations was taking into account a variety of contributing factors. Among them, the energy terms associated with bending of microtubules and with osmotic maintenance of the cell volume were found particularly important. When the centrosome adopts varying positions in the cell, one side of which is flattened, the microtubules have to bend differently in order to “fit” inside the given cell volume. These differences provide one source of the energy gradient, which favors that position of the centrosome which is also biologically functional. This numerical finding may appear mystical, unless it is reformulated in a more evolutionary way: the position that is favored by the physical forces that are inherent in the basic cell structure becomes also the one that acquires function in development and immunity—organism-level evolution makes use of the material that is provided by cell-level physics.
Like pulling, the energy minimization is an error-prone mechanism. The calculations reveal a rugged, pitted energy landscape, in which the global minimum corresponding to the biologically functional centrosome orientation is surrounded by multiple local minima, where the system may “get stuck” on the way down the energy gradient, without ever reaching the global minimum.Citation27 These computational findings may explain why redundancy appears to be sought by the cell, P1 or T. The overall reliability of centrosome positioning, in the face of the biophysical constraints on each mechanism, should be increased when both are deployed in parallel. Considering the complexity that seems to permeate the centrosome positioning mechanics, however, it remains to be examined if this conjecture can stand the test of rigorous analysis.
Conclusions
In terms of comparative systems biomechanics, the reviewed studies demonstrate that the P and T cells represent a relatively general and complex case of centrosome positioning. Another system in which spindle positioning has been elucidated by means of quantitative biophysical analysis is the spindle orientation in highly transformed cultured HeLa cells. These cells round up completely during mitosis, thus erasing the cell shape effects and revealing in their pure form the effects of localized cortical pulling.Citation39,Citation40 Compared with flat interphase cells of typical cultured lines, and compared with the rod-like yeast cells,Citation41 in which microtubules stretch essentially in one or two dimensions, the microtubule cytoskeletons in P and T cells are truly three-dimensional. Not unexpectedly, this makes their mechanical properties more complex than in the one-dimensional case, rendering the centered equilibrium unstable. Less intuitively, their theoretical mechanics is simpler than in the highly flattened cells, on which much of the research into the molecular regulation of centrosome positioning has been conducted.Citation15 Confinement to two dimensions “traps” metastable equilibrium forms of microtubules, which are unstable and therefore not observed in the voluminous cells. Their presence in flat cells predicts that the centrosome positioning should be irreversible and the equilibrium dependent on past transformations.Citation29 Overall, we can tentatively posit that Caenorhabditis elegans blastomeres and human lymphocytes represent close and intermediate levels of biomechanical complexity, whose spectrum we are only beginning to explore.
Figures and Tables
Figure 1 Schematic of centrosome positioning in Caenorhabditis elegans blastomeres and T lymphocytes. (A) single-cell stage of the worm embryo. (B) two-cell stage of the embryo. (C) isolated T lymphocyte, a white blood cell in suspension. (D) T cell conjugated with an antigen-presenting cell. Cell boundaries are in black, centrosomes in red and microtubules in blue. Gray arrows show translational, rotational and oscillatory movements.
Acknowledgements
This work was supported by grant GM078332 from the US National Institutes of Health.
Addendum To:
References
- Schatten H. The mammalian centrosome and its functional significance. Histochem Cell Biol 2008; 129:667 - 686
- Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nat Cell Biol 2009; 11:365 - 374
- Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol 2008; 9:1105 - 1111
- Hyman AA, White JG. Determination of cell division axes in the early embryogenesis of Caenorhabditis elegans. J Cell Biol 1987; 105:2123 - 2135
- Albertson DG. Formation of the first cleavage spindle in nematode embryos. DevBiol 1984; 101:61 - 72
- Laufer J, Bazzicapuso P, Wood WB. Segregation of development potential in early embryos of Caenorhabditis elegans. Cell 1980; 19:569 - 577
- Strome S, Wood W. Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell 1983; 35:15 - 25
- Keating HH, White JG. Centrosome dynamics in early embryos of Caenorhabditis elegans. J Cell Sci 1998; 111:3027 - 3033
- Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol 1982; 95:137 - 143
- Kupfer H, Monks CKF, Kupfer A. Small splenic B cells that bind to antigen-specific T helper (Th) cells and face the site of cytokine production in the Th cells selectively proliferate: immunofluorescence microscopic studies of Th-B antigen-presenting cell interactions. J Exp Med 1994; 179:1507 - 1515
- Bykovskaja SN, Rytenko AN, Rauschenbach MO, Bykovsky AF. Ultrastructural alteration of cytolytic T lymphocytes following their interaction with target cells: I. Hypertrophy and change of orientation of the Golgi apparatus. Cell Immunol 1978; 40:164 - 174
- Kupfer A, Swain SL, Janeway C Jr, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. Proc Natl Acad Sci USA 1986; 83:6080 - 6083
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 2006; 443:462 - 465
- Kuhn JR, Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity 2002; 16:111 - 121
- Manneville JB, Etienne-Manneville S. Positioning centrosomes and spindle poles: looking at the periphery to find the centre. Biol Cell 2006; 98:557 - 565
- Grill SW, Kruse K, Jülicher F. Theory of mitotic spindle oscillations. Phys Rev Lett 2005; 94:108104
- Pecreaux J, Röper JC, Kruse K, Jülicher F, Hyman AA, Grill SW, et al. Spindle oscillations during asymmetric cell division require a threshold number of active cortical force generators. Curr Biol 2006; 16:2111 - 2122
- Kozlowski C, Srayko M, Nedelec F. Cortical microtubule contacts position the spindle in C. elegans embryos. Cell 2007; 129:499 - 510
- Grill SW, Hyman AA. Spindle positioning by cortical pulling forces. Dev Cell 2005; 8:461 - 465
- Grill SW, Gönczy P, Stelzer EH, Hyman AA. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 2001; 409:630 - 633
- Redemann S, Pecreaux J, Goehring NW, Khairy K, Stelzer EHK, Hyman AA, et al. Membrane invaginations reveal cortical sites that pull on mitotic spindlesin one-cell C. elegans embryos. PLoS ONE 2010; 5:12301
- Kim MJ, Maly IV. Deterministic mechanical model of T-killer cell polarization reproduces the wandering of aim between simultaneously engaged targets. PLoS Comput Biol 2009; 5:1000260
- Combs J, Kim SJ, Tan S, Ligon LA, Holzbaur EL, Kuhn J, et al. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci USA 2006; 103:14883 - 14888
- Martín-Cófreces NB, Robles-Valero J, Cabrero JR, Mittelbrunn M, Gordón-Alonso M, Sung CH, et al. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol 2008; 182:951 - 962
- Grill SW, Howard J, Schäffer E, Stelzer EHK, Hyman AA. The distribution of active force generators controls mitotic spindle position. Science 2003; 301:518 - 521
- Schmidt DJ, Rose DJ, Saxton WM, Strome S. Functional analysis of cytoplasmic dynein heavy chain in Caenorhabditis elegans with fast-acting temperature-sensitive mutations. Mol Biol Cell 2005; 16:1200 - 1212
- Arkhipov SN, Maly IV. Contribution of whole-cell optimization via cell body rolling to polarization of T cells. Phys Biol 2006; 3:209 - 219
- Baratt A, Arkhipov SN, Maly IV. An experimental and computational study of effects of microtubule stabilization on T-cell polarity. PLoS ONE 2008; 3:3861
- Maly VI, Maly IV. Symmetry, stability and reversibility properties of idealized confined microtubule cytoskeletons. Biophys J 2010; 99:2831 - 2840
- Holy TE. Physical aspects of the assembly and Function of Microtubules Dissertation Princeton University 1997;
- Bjerknes M. Physical theory of the orientation of astral mitotic spindles. Science 1986; 234:1413 - 1416
- Skop AR, White JG. The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr Biol 1998; 8:1110 - 1116
- Hyman AA. Centrosome movement in the early divisions of Caenorhabditis elegans: A cortical site determining centrosome position. J Cell Biol 1989; 109:1185 - 1193
- Tsou MFB, Ku W, Hayashi A, Rose LS. PAR-dependent and geometry-dependent mechanisms of spindle positioning. J Cell Biol 2003; 160:845 - 855
- Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 1995; 83:743 - 752
- Arkhipov SN, Maly IV. Retractile processes in T lymphocyte orientation on a stimulatory substrate: morphology and dynamics. Phys Biol 2008; 5:16006
- Arkhipov SN, Maly IV. Quantitative analysis of the role of receptor recycling in T cell polarization. Biophys J 2006; 91:4306 - 4316
- Arkhipov SN, Maly IV. A model for the interplay of receptor recycling and receptor-mediated contact in T cells. PLoS ONE 2007; 2:633
- Théry M, Racine V, Pépin A, Piel M, Chen Y, Sibarita JB, et al. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol 2005; 7:947 - 953
- Théry M, Jiménez-Dalmaroni A, Racine V, Bornens M, Jülicher F. Experimental and theoretical study of mitotic spindle orientation. Nature 2007; 447:493 - 496
- Tran PT, Marsh L, Doye V, Inoué S, Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J Cell Biol 2001; 153:397 - 412