Abstract
The vacuole is likely to play a variety of roles in supporting host colonization and infection by pathogenic species of fungi. In the human pathogen Candida albicans, the vacuole undergoes dynamic morphological shifts during the production of the tissue invasive hyphal form, and this organelle is required for virulence. Recent efforts in my lab have focused on defining which vacuolar trafficking pathways are required for C. albicans hyphal growth and pathogenesis. Our results indicate that there are several distinct trafficking routes between the Golgi apparatus and vacuole. However, there is a large degree of functional overlap between each with respect to their roles in hyphal growth and virulence. Herein we consider these results and propose that during hyphal growth, specific trafficking routes maybe less important than the aggregate vacuolar trafficking capacity.
The fungal vacuole is typically a large organelle with major roles in the degradation of cellular macromolecules, storage of nutrients and stress tolerance.Citation1 Several recent studies have clearly established that this organelle plays a crucial role in supporting host invasion by both mammalian and plant fungal pathogens.Citation2 Our studies with the human pathogen, Candida albicans, have shown that the vacuole, is crucial for stress tolerance, polarized hyphal growth and virulence.Citation3–Citation5 However, the specific mechanisms which support pathogenesis remain unknown. C. albicans hyphal growth facilitates tissue injury, may enable escape from phagocytic cells and is required for virulence.Citation6–Citation8 Thus the C. albicans vacuole may support pathogenesis on two levels: (1) to promote stress resistance required for survival within the hostile host; and (2) to support tissue invasive hyphal growth. Importantly, the fungal vacuole has diverged significantly from the analogous mammalian lysosome.Citation1,Citation9 Thus the fungal specific functions of the vacuole may provide a basis to selectively target pathogenic fungi with chemo-therapeutic agents. The requirement of this organelle for C. albicans virulence makes it an especially attractive target for developing new antifungals. In order to exploit this organelle as a therapeutic strategy, it will be necessary to define the precise functions, pathways and components required to support pathogenesis.
Vacuolar Trafficking during Hyphal Growth
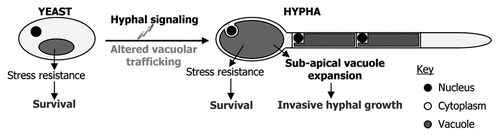
Hyphal growth involves established mechanisms, including the induction of a hyphal specific transcription program; cytoskeletal polarization; and localized exocytosis at the hyphal apex. Intriguingly, during C. albicans hyphal growth, subapical vacuoles dramatically expand to produce cells devoid of cytoplasm, while the cytoplasm migrates in the apical compartment ().Citation10–Citation12 This “vacuolation” of distal hyphal cells necessitates a significant redistribution of cellular membrane to sub-apical vacuoles, however, the molecular mechanisms underlying this process and its regulation, remain undetermined. Furthermore, it is unclear how such dynamic cytological shifts may support polarized hyphal growth. It is possible that vacuolar expansion permits rapid hyphal elongation while reducing the requirement for cytoplasmic biosynthesis. Alternatively, this may generate turgor pressure to drive apical extension.
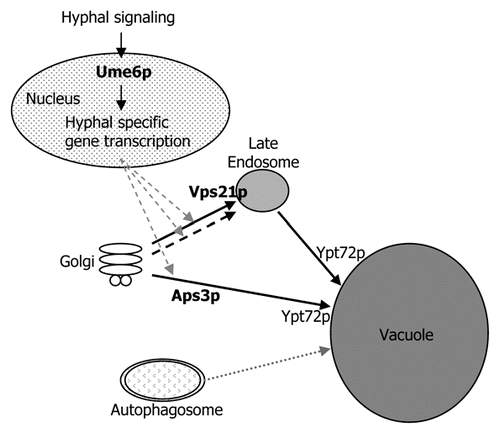
The non-pathogenic (and non-hyphal) yeast Saccharomyces cerevisiae has multiple vacuolar trafficking pathways.Citation13 Membrane trafficking from the Golgi apparatus is crucial for vacuole biogenesis and occurs via at least two distinct pathways; a direct route facilitated by the AP-3 coat complex (including Aps3p); and an indirect route via the late endosome, which is dependent upon the Vps21p GTPase. The goal of my recent research paper,Citation14 was to investigate the importance of these two pathways with respect to C. albicans hyphal growth and virulence. C. albicans homologs of APS3 and VPS21 were identified and gene deletion strains constructed. Loss of VPS21 mediated trafficking causes mild reductions in C. albicans stress resistance, hyphal growth and virulence, however, loss of APS3 has no detectable affect on these phenotypes.Citation14 Strikingly, loss of both VPS21 and APS3 causes a synthetic stress phenotype, as well as profound defects in hyphal growth and virulence. This suggests that these two distinct trafficking routes are able to partially compensate for each other with respect to their roles in stress tolerance, hyphal growth and virulence.
Regulation of Candida albicans Vacuolar Trafficking
Inducing C. albicans hyphal growth requires shifts in medium and temperature. Inducing signals are detected and propagated via well defined signaling pathways, which induce a hyphal specific transcriptional program through transcription factors including Ume6p.Citation15 Many hyphal inducing media are nutrient poor, and may induce stress responses. Thus the defective stress responses of the double mutant could indirectly account for the defective hyphal growth. Ume6p overexpression bypasses the hyphal signaling pathways to cause constitutive hyphal growth.Citation16 This enables induction of hyphal growth under stress free conditions. Our studies have shown that Ume6p overexpression in wild-type C. albicans, is sufficient not only for constitutive hyphal growth but also sub-apical vacuolation.Citation14 Thus the mechanisms which facilitate sub-apical vacuolation are controlled directly or indirectly by Ume6p dependent transcriptional responses. Moreover, Ume6p coordinately regulates apical extension and sub-apical vacuolation. Ume6p overexpression also induced hyphal growth and sub-apical vacuolation in the aps3Δ/Δ and vps21Δ/Δ single mutants, but the double mutant had profound defects in polarized hyphal growth and failed to form the vacuolated sub-apical compartments. This established that Golgi to vacuole trafficking is required to support Ume6p induced hyphal growth, with membrane trafficking through the Aps3p and Vps21p dependent pathways being largely redundant. This also supports that the stress phenotypes are unlikely to account for the defective hyphal growth when Golgi to vacuolar trafficking is disrupted.
How Ume6p regulated transcriptional responses alter vacuolar trafficking during the transition from yeast to hyphal growth remains to be established. We propose that hyphal signaling through Ume6p, stimulates Golgi to vacuole membrane trafficking through the endosomal and AP-3 pathways to promote sub-apical vacuole expansion, and thus support apical extension ().
Redundancy of Vacuolar Trafficking Routes
Surprisingly, the aps3Δ/Δvps21Δ/Δ mutant has a largely intact vacuole in the yeast form. Furthermore, most of a vacuolar membrane protein, Mlt1p, still reaches the vacuole and the vacuole is acidified, indicating normal trafficking of the vacuolar ATPase.Citation14 This suggests that additional pathways are able to deliver vacuolar proteins from the Golgi and are sufficient to maintain vacuole morphology in the yeast form, but insufficient to support hyphal growth or virulence. We have recently focused upon a GTPase, which is closely related to and co-localizes with Vps21p, and may also function in regulating endosomal trafficking (unpublished results). The apparent redundancy and multiple trafficking routes between the Golgi and vacuole may suggest that specific pathways are less important than the collective membrane trafficking capacity to the vacuole. Furthermore, there may be a “threshold” of membrane transport that is required to sustain polarized hyphal growth, with each route making a contribution to the overall trafficking “volume.” In this case, progressive loss of individual trafficking routes would progressively inhibit hyphal growth due to a decreased ability to evacuate the cytoplasm from sub-apical cells. Thus loss of Aps3p and Vps21p pathways is insufficient to block vacuolar biogenesis in the yeast form, but drops the trafficking threshold below that required to effectively expand sub-apical vacuoles during hyphal growth, in turn limiting apical extension. Accordingly, it will be important to determine the contribution of each individual vacuolar trafficking route to hyphal growth and virulence.
Summary and Future
It is clear that the fungal vacuole plays an essential role in supporting fungal pathogenesis and this large, multifunctional organelle is likely to perform a variety of important functions during host colonization and infection.
My laboratory has analyzed the contribution of several distinct vacuolar trafficking pathways to C. albicans hyphal growth and virulence.Citation3,Citation14,Citation17 However, the mechanism by which vacuolar trafficking supports invasive hyphal growth remains to be determined. It will be important to examine if there is functional interdependency between the polarisome and the sub-apical vacuoles or if apical organization and sub-apical vacuolation occur via completely independent mechanisms. More specifically, does loss of vacuolar integrity or trafficking impact actin polarization or polarisome organization at the hyphal apex? An important step in exploring the potential utility of this organelle as a target for antifungal therapy, will be to identify specific vacuolar pathways or functions which promote pathogen survival and invasion of host tissue.
Abbreviations
| AP-3 | = | adaptor protein complex 3 |
Figures and Tables
Figure 2 Vacuolar trafficking and regulation in Candida albicans. Our results have established that both the Aps3p (AP-3) and Vps21p (endosomal) dependent vacuolar trafficking routes contribute to hyphal growth, presumably by facilitating the vacuolation of sub-apical cells. Our data also suggest a second Golgi→endosome→vacuole trafficking route exists (dashed line), facilitated by a GTPase closely related to Vps21p. While loss of either pathway alone results in minor defects in hyphal growth, loss of more than one seems to lead to severe defects. However, our results have not demonstrated a role for autophagy in C. albicans hyphal growth or virulence (dotted line).Citation17 We suggest that activation of a hyphal specific transcriptional program through the Ume6p transcription factor, stimulates vacuolar trafficking through AP-3 and endosomal pathways to facilitate sub-apical vacuolation.
Addendum to:
References
- Klionsky DJ, Herman PK, Emr SD. The fungal vacuole: composition, function and biogenesis. Microbiol Rev 1990; 54:266 - 292
- Veses V, Richards A, Gow NA. Vacuoles and fungal biology. Curr Opin Microbiol 2008; 11:503 - 510
- Johnston DA, Eberle KE, Sturtevant JE, Palmer GE. Role for endosomal and vacuolar GTPases in Candida albicans pathogenesis. Infect Immun 2009; 77:2343 - 2355
- Palmer GE, Cashmore A, Sturtevant J. Candida albicans VPS11 is required for vacuole biogenesis and germ tube formation. Eukaryot Cell 2003; 2:411 - 421
- Palmer GE, Kelly MN, Sturtevant JE. The Candida albicans vacuole is required for differentiation and efficient macrophage killing. Eukaryot Cell 2005; 4:1677 - 1686
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell 1997; 90:939 - 949
- Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 2004; 3:1076 - 1087
- Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2003; 2:1053 - 1060
- Armstrong J. Yeast vacuoles: more than a model lysosome. Trends Cell Biol 2010; 20:580 - 585
- Gow NA, Gooday GW. A model for the germ tube formation and mycelial growth form of Candida albicans. Sabouraudia 1984; 22:137 - 144
- Gow NA, Gooday GW. Vacuolation, branch production and linear growth of germ tubes in Candida albicans. J Gen Microbiol 1982; 128:2195 - 2198
- Barelle CJ, Bohula EA, Kron SJ, Wessels D, Soll DR, Schafer A, et al. Asynchronous cell cycle and asymmetric vacuolar inheritance in true hyphae of Candida albicans. Eukaryot Cell 2003; 2:398 - 410
- Bowers K, Stevens TH. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 2005; 1744:438 - 454
- Palmer GE. Endosomal and AP-3-dependent vacuolar trafficking routes make additive contributions to Candida albicans hyphal growth and pathogenesis. Eukaryot Cell 2010; 9:1755 - 1765
- Banerjee M, Thompson DS, Lazzell A, Carlisle PL, Pierce C, Monteagudo C, et al. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol Biol Cell 2008; 19:1354 - 1365
- Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, Lopez-Ribot JL, Kadosh D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci USA 2009; 106:599 - 604
- Palmer GE, Kelly MN, Sturtevant JE. Autophagy in the pathogen Candida albicans. Microbiology 2007; 153:51 - 58