Abstract
Mitogen-activated protein kinase (MAPK) signaling influences a variety of neuronal properties, including structural characteristics such as spine density and physiological features like long-term potentiation. Spatiotemporal control of MAPK signalling is crucial to generate specific changes in neuronal physiology. However, while many studies have concentrated on the activation of MAPK signaling by trophic factors such as BDNF and neuronal activity, the mechanisms that lead to its termination have not been well described. Two recent reports begin to address this question by focusing on the role of the MAPK phosphatase, MKP-1, in neuronal function. The first study provides a cellular mechanism underlying MKP-1 action in the brain.1 The second study describes potential roles of MKP-1 during stress and major depression.2
Although the role of MAPK phosphatase, MKP-1, in immune responses and cancer is well documented,Citation3,Citation4 its function in the nervous system has remained largely unknown. MKP-1 is a phosphatase that belongs to the dual specificity phosphatase (DUSP) family. DUSPs are conserved across species from yeast to plants to humans.Citation5 A unique feature of DUSPs is to dephosphorylate both the tyrosine and threonine residues within a conserved TxY motif,Citation4 a consensus sequence found in the catalytic core of MAPKs. Phosphorylation of the TxY motif at both residues is required for full MAPK activity.Citation4 MAPKs integrate extracellular signals (e.g., neurotrophins, morphogens, neurotransmitters) to initiate intracellular programs involved in cell differentiation, survival, apoptosis and synaptic plasticity.Citation6 The strength of MAPK signaling frequently determines distinctive outcomes through the duration of its activities.Citation7,Citation8 Of the many MAPK members, MKP-1 tends to dephosphorylate and inactivate specific members, with a preference for p38 and JNK and, to a lesser extent, Erk1/2.Citation9
Expression of mkp-1 is driven by neural activity in the primary sensory areas of the brain and is frequently observed with the neurotrophin BDNF.Citation10,Citation11 Visual stimuli, songs and motor tasks robustly increase MKP-1 transcripts in visual, auditory and somatosensory neurons, respectively.Citation10,Citation12,Citation13 Conditional loss of BDNF in the prefrontal cortex resulted in a dramatic decrease in mkp-1 transcripts,Citation14 suggesting that BDNF can regulate the levels of MKP-1 gene products. Consistent with several in vivo observations, we found that MKP-1 protein is expressed at very low levels in cultured developing inhibitory and excitatory neurons, but is highly induced after BDNF treatment and neuronal activity.Citation1 Like other immediate early genes, mkp-1 expression is labile, with low baseline levels, rapid induction and turnover that is responsive to different cellular signals.Citation15 Indeed, turnover of MKP-1 protein is extremely rapid ( and right), but is delayed by BDNF signaling ( and left),Citation1 via a sophisticated phosphorylation/ubiquitination regulatory system ().
One consequence of transient ectopic expression of MKP-1 in the developing cortex is aberrant axon branching. In contrast, loss of MKP-1 resulted in an inability of BDNF and neuronal activity to produce new axon branches.Citation1 Hence, regulation of MKP-1 levels by trophic factors and neuronal stimuli can result in changes in axon arborization. BDNF is well known for its effects upon axon outgrowth and branching.Citation16 Indeed, BDNF signaling from the distal axon can induce MKP-1 activity in the cell body and the axon. The regulation of MKP-1 expression by BDNF provides a significant mechanism to account for how neuronal networks may be refined in an activitydependent manner.
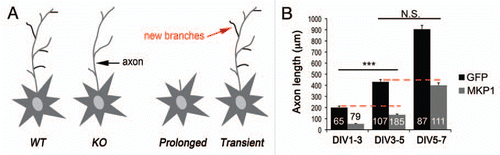
In cortical neurons, MKP-1 deactivates JNK, a MAP kinase member that is constitutively active in axons. Dephosphorylation of JNK by MKP-1 perturbed axon growth, whereas constitutive JNK activity could overcome axonal defects caused by prolonged expression of MKP-1. What is the consequence of an axonal loss of JNK activity? One effect is a decrease in microtubule stability that is promoted by post-translational modifications of proteins, such as tubulin.Citation17 Our study indicates that by deactivating JNK, MKP-1 decreases the phosphorylation of key axonal JNK substrates (e.g., stathmins, tau, neurofilaments), leading to microtubule destabilization.Citation1,Citation18 The longer MKP-1 is expressed, the stronger the JNK dephosphorylation and microtubule destabilization, translating in axonal shape changes from enhanced branching to degeneration of the axon ( and B).
The tight control of MKP-1 protein turnover by phosphorylation and ubiquitination provides a useful mechanistic framework to study the regulation of intracellular signaling events by synaptic activity. Indeed, Erk1/2, which is activated by focal BDNF signaling, is a kinase for and a non-preferred substrate of MKP-1. Inhibition of Erk1/2 accelerates the turnover of MKP-1 by preventing its phosphorylation at defined residues at the C-terminus.Citation1 With low basal expression, high inducibility, rapid turnover and the opportunity for stabilization, MKP-1 displays the necessary attributes to control MAPK signaling and cytoskeletal remodelling temporally at defined cellular locations. As the strength of MAPK signaling determines the duration of biological responses and many physiological outcomes, spatio-temporal titration of MKP-1 plays a pivotal regulatory role. It is tempting to speculate that stabilization of MKP-1 protein levels by BDNF/Erk1/2 signaling may instruct and determine precise axonal branching and connectivity.
If titration of MKP-1 levels is important for neuronal signal transduction, what may result from the deregulation of its expression during higher order behaviors? Enhanced expression of MKP-1 has recently been reported in postmortem hippocampal tissues from individuals with major depression.Citation2 Induction of mkp-1 in the hippocampus also occurs in animal models following chronic severe stress or seizures.Citation2,Citation19 Whether upregulation of MKP-1 is a cause or consequence of these conditions is presently unknown. However, experimental elevation of MKP-1 levels in the hippocampus produced depressive-like effects in absence of stress.Citation2 At the cellular level, neither overexpression nor genetic ablation of MKP-1 affected neuronal survival, but potentially could impair cell differentiation. Indeed, we found that prolonged ectopic expression of MKP-1 impedes axonal growth at many stages of neuronal development (). If the chronic misexpression of MKP-1 contributes to the gradual development of depression, then restoring baseline MKP-1 levels may prove beneficial. Two lines of evidence support this idea. First, the persistent overexpression of MKP-1 induced by chronic stress is reversed by antidepressant treatment.Citation2 Second, mkp-1 knock out mice are resilient to severe stressful experiences compared to wildtype littermates. Therefore, if the molecular mechanisms that disrupt expression of mkp-1 are causal to mood disorders, transient antagonism of MKP-1 phosphatase activity may represent therapeutic value.
Abbreviations
| MAPK | = | mitogen-activated protein kinase |
| MKP | = | MAPK phosphatase |
| BDNF | = | brain-derived neurotrophic factor |
| ERK | = | extracellular related kinase |
| JNK | = | c-jun N-terminal kinase |
Figures and Tables
Figure 1 Control of MKP-1 levels. (A) Baseline expression of MKP-1 is low, but highly inducible by sensory stimuli, neuronal activity and trophic factors such as BDNF. Once expressed, MKP-1 is unstable with a turnover <1 h. When phosphorylated by neuronal activity or BDNF, MKP-1 stability extends to >8 h.Citation1 As a consequence, MAPK signaling is shaped accordingly to MKP-1 levels. (B) The mechanisms that regulate MKP-1 levels involve protein kinases and ubiquitin ligases. Abnormal expression of MKP-1 is a hallmark of several disorders, such as stress and depression.Citation2
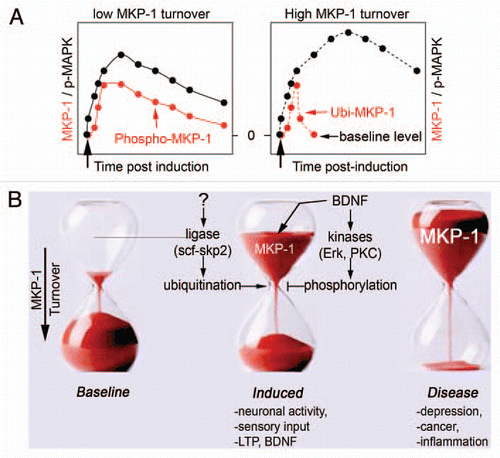
Figure 2 Titration of MKP-1 levels determines the physiological outcome. (A) Scheme summarizing the effects of MKP-1 on morphological plasticity. Loss of mkp-1 prevents activity-dependent and BDNF-induced morphological plasticity of the axon compared to wildtype controls. However, neuronal morphology is indistinguishable between the two genotypes under baseline conditions. Transient induction of MKP-1 mirrors the effects of BDNF and neuronal activity on axon branching, whereas prolonged expression prevents axon growth. (B) Ectopic expression of MKP-1 disrupts axonal growth at any stage of development. Length of the longest neurite per cell (mean ± SEM). Numbers in bars indicate the number of cells analyzed. ***p < 0.01, t test, n= 3 independent experiments. n.s., not significant.
Addendum To:
References
- Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci 2010; 13:1373 - 1379
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, et al. A negative regulator of MAP kinase causes depressive behavior. Nat Med 2010; 16:1328 - 1332
- Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases—regulating the immune response. Nat Rev Immunol 2007; 7:202 - 212
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death and cancer. Pharmacol Rev 2008; 60:261 - 310
- Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature 1992; 359:644 - 647
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem 2001; 76:1 - 10
- Chao MV. Growth factor signaling: where is the specificity?. Cell 1992; 68:995 - 997
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 1995; 80:179 - 185
- Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 2007; 26:3203 - 3213
- Horita H, Wada K, Rivas MV, Hara E, Jarvis ED. The dusp1 immediate early gene is regulated by natural stimuli predominantly in sensory input neurons. J Comp Neurol 2010; 518:2873 - 2901
- Genoud C, Knott GW, Sakata K, Lu B, Welker E. Altered synapse formation in the adult somatosensory cortex of brain-derived neurotrophic factor heterozygote mice. J Neurosci 2004; 24:2394 - 2400
- Chen MF, Chen HI, Jen CJ. Exercise training upregulates macrophage MKP-1 and affects immune responses in mice. Med Sci Sports Exerc 2010; 42:2173 - 2179
- Doi M, Cho S, Yujnovsky I, Hirayama J, Cermakian N, Cato AC, et al. Light-inducible and clock-controlled expression of MAP kinase phosphatase1 in mouse central pacemaker neurons. J Biol Rhythms 2007; 22:127 - 139
- Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA, et al. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry 2006; 11:633 - 648
- Zeitlinger J, Stark A, Kellis M, Hong JW, Nechaev S, Adelman K, et al. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat Genet 2007; 39:1512 - 1516
- Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science 2002; 298:770 - 776
- Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 2009; 10:319 - 332
- Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev 2006; 70:1061 - 1095
- Qian Z, Gilbert M, Kandel ER. Temporal and spatial regulation of the expression of BAD2, a MAP kinase phosphatase, during seizure, kindling and long-term potentiation. Learn Mem 1994; 1:180 - 188