 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The bacterium Caulobacter crescentus shows a remarkable spatial ordering of its chromosome that leads to a strong linear correlation between the position of genes on the chromosomal map and their spatial position in the cellular volume. In a recent study we have shown that a robust and universal geometrical ordering mechanism can explain this correlation. We demonstrated that self-avoidance of DNA, specific positioning of one or few DNA loci (such as origin or terminus) together with the action of DNA compaction proteins (that organize the chromosome into topological domains) are sufficient to get a linear arrangement of the chromosome along the cell axis. This configuration, however, only represents the population average. Individual cells can have DNA arrangements that deviate significantly from the mean configuration and that break left-right symmetry. Symmetry breaking is stronger for longer chromosomes.
Recently it has been experimentally demonstrated that the genome of the bacterium C. crescentus has a highly regular spatial structure.Citation1 In swarmer cells (that are in the non-replicating G1 state) origin (ori) and terminus (ter) are positioned at opposite cell poles. The intervening chromosomal loci show a strong linear correlation between their position on the chromosomal map and their position in the subcellular volume. Similar arrangements have been found in E. coli cells.Citation2 However, here dynamics and organization of the chromosome are more complex and growth-phase dependent.Citation3–Citation9 Typically, localization patterns with ori and ter at opposite poles are only found in newborn cells.Citation10,Citation11
In a recent study,Citation12 we have theoretically analyzed the basis of chromosomal organization in bacteria. We demonstrated that confinement of chromosomal domains to specific cellular positions has a strong influence on the spatial arrangement of the chromosome in the cell. In particular, we found that positioning of ori and ter to opposite cell poles in C. crescentus gives rise to the striking linear correlation found in reference Citation1. For E. coli we made predictions about the growth-stage dependence of the spatial arrangement of the chromosome. The conclusion were drawn from a theoretical model with the following main ingredients:
All cells have a single chromosome of fixed length that lies inside the prescribed cellular volume. The origin and terminus have fixed spatial positions.
The cellular volume is represented by a three-dimensional cubic lattice. The chromosome is represented by a self-avoiding random walk on this lattice.
Each step of the random walk represents a compacted unit of the chromosome.
Compaction is the key ingredient of our model that is required to obtain the experimentally observed linear correlation. The specific scenario that we have in mind is that compaction proteins (such as H-NS, HU, FIS and TktACitation13) locally compact the chromosome giving it the shape of a chain of spheres (i.e., “blobs”) with a typical diameter db ≈ 30 nm. Each step of the random walk represents such a blob. From the measurements of reference 14 we concluded that there are ∼2,000 of these compacted units. A similar description of the chromosome was recently developed by Jun and coworkers in the context of chromosomal segregation.Citation15 These authors also provide more details about molecular mechanisms that could give rise to such a blob-like chromosomal arrangement.Citation16
The big advantage of our model is that it does not depend on the details on how these blobs are formed or even what they correspond to; the only requirement is that the blobs effectively reduce the length of the random walk. In particular, our main conclusions are independent of the specific mechanism that gives rise to the compact structure.
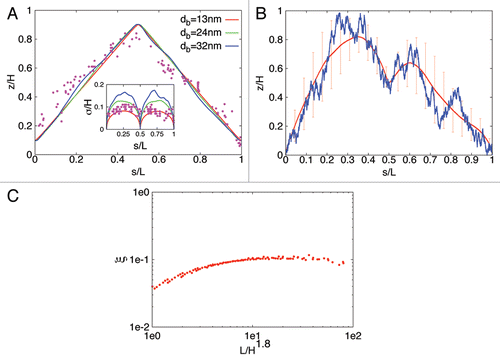
We analyzed our model by generating ensembles of bacterial DNA configurations with extensive stochastic Monte Carlo computer simulations. A typical result of our model is shown in . As can be seen the model reproduces the experimental results quite well (possible causes of the small differences close to the ori pole are discussed in ref. Citation12). Similar results were found for newborn E. coli cells. However, here the DNA configurations depend on growth stage and in particular upon initiation of replication different arrangements are found, for details see reference Citation12. In all cases, the theoretical results do not require a fine-tuning of the parameters but rather are very robust for a large range of parameter values. In particular:
The correlation between spatial and genome localization is nearly perfectly linear for sufficiently large blob diameters (i.e., db ≥ 24 nm for C. crescentus and db ≥ 75 nm for E. coli).
The linear correlation also holds for a large range of blob numbers: for C. crescentus for 200 to 2,000 blobs, for E. coli for 200 to 600 blobs.
Linear DNA configurations are also found in a large range of cell volumes. This is important for E. coli that shows a ∼10-fold change in volume with growth rate.
With increasing DNA content the linear arrangement of the chromosome becomes stronger. Furthermore, the geometrical ordering also works for a large range of chromosome lengths (ranging from L = 1.5 mm to 3 mm) indicating that our proposed mechanism is applicable to different bacteria.
There are also more general conclusions that can be drawn from our analysis. The spatial chromosomal arrangement is quite robust with respect to variations in the positioning of ori and ter. In fact, linear configurations are also found if only ori has a fixed position. In this case ter is free to move but the remaining parts of the chromosome confine its spatial position in this way effectively fixing its position. This implies, that even though ter appears in vivo at a specific position one cannot conclude that this position is fixed by, e.g., anchoring to the pole. Interestingly, an anchoring mechanism for ter has so far not been identified (while it is known that ori is anchored to the flagellated pole by PopZCitation17,Citation18).
A model that includes only self-avoidance (that, for example, could be induced by electrostatic repulsion between the DNA) but not a mechanism that effectively compacts the chromosome cannot explain the linear correlation. Thus, (sufficiently strong) compaction is essential. This effect cannot be due to supercoiling alone making the action of compaction proteins the most plausible scenario. The importance of compaction was recently also demonstrated in other approaches.Citation2,Citation19
DNA organization is a stochastic process that leads to cell-to-cell variations in the chromosomal arrangement. The linear configuration shown in corresponds to the average configuration of the population. Individual cells can have realizations that deviate quite significantly from this population mean (as indicated by the large standard deviations from the mean curve in ). In fact, individual cells can even have asymmetric DNA configurations, where, e.g., the left strand is closer to the ter pole and the right strand is closer to the ori pole, see . The opposite configuration (with the left [right] strand closer to the ori [ter] pole) occurs with the same probability so that in the population the average configuration is perfectly symmetric. The strength of the symmetry breaking in individual cells can be quantified with the following order parameter
1
1
In the last two equations zj denotes the height of blob j belonging to the left respectively to the right chromosomal strand. The time-average of this quantity is shown in as function of chromosomal length L for different cell volumes H × H × H. Interestingly, all points calculated from EquationEquation 11
1 for the different combinations of L and H collapse onto a single curve if plotted as function of L/H1.8. As one can see symmetry breaking is stronger for longer chromosomes or smaller cells. If the linear organization of the chromosome has a physiological role then one expects that additional mechanisms (such as anchoring of more chromosomal loci) are required to suppress these asymmetric configurations.
Figures and Tables
Figure 1 Average subcellular position of genes as function of their position on the chromosome in C. crescentus and E. coli as obtained from numerical simulations of compacted DNA . (A and B) show the position of genes along the cell axis as function of their position on the chromosomal map for an average chromosome configuration as obtained from our theoretical model in which compacted DNA is represented by a chain of blobs. The position on the chromosome is parameterized by the contour length s (measured in units of DNA length L). The configurations shown in (A) are for different blob diameters but in all cases 2,000 blobs are used to represent the chromosome (of length 4.02 Mbp) of a C. crescentus swarmer cell. The implemented cell volume has height H = 2 µm and cross section 0.5 µm × 0.5 µm. The fixed positions of ori and ter (at zori = 0.1H and zter = 0.9H) have been adjusted to minimize the differences between the experimental data of reference 1 (dots) and the predictions of the model. (B) shows a symmetry-breaking chromosomal configuration (blue line) in an individual E. coli cell (with volume 1 µm × 1 µm × 1 µm). The red line represents the average symmetry-breaking configuration (averaged over 50,000 chromosomal arrangements). Bars indicate the standard deviation from the mean configuration. In the configurations shown ori is at the cell pole and ter is positioned in midcell (zori = 0.1H and zter = 0.5H). The chromosome has a length of 11,310 blobs. Asymmetric chromosomal arrangements occur also for different ori and ter positions. (C) shows the averaged order parameter ξ for cells with volume H × H × H and chromosome length L. The chromosomal arrangements are more asymmetric for longer chromosomes (or smaller cells). The data shown are for zori = 0 and zter = H.
Addendum to:
References
- Viollier PH, Thanbichler M, McGrath PT, West L, Meewan M, McAdams HH, et al. Rapid and sequential movement of individual chromosomal loci to specific subcellular locations during bacterial DNA replication. Proc Natl Acad Sci USA 2004; 101:9257 - 9562
- Wiggins PA, Cheveralls KC, Martin JS, Lintner R, Kondev J. Strong intranucleoid interactions organize the Escherichia coli chromosome into a nucleoid filament. Proc Natl Acad Sci USA 2010; 107:4991 - 4995
- Toro E, Shapiro L. Bacterial chromosome organization and segregation. Cold Spring Harb Perspect Biol 2010; 2:349
- Sherratt DJ. Bacterial chromosome dynamics. Science 2003; 301:780 - 785
- Nielsen HJ, Ottesen JR, Youngren B, Austin SJ, Hansen FG. The Escherichia coli chromosome is organized with the left and right chromosome arms in separate cell halves. Mol Microbiol 2006; 62:331 - 338
- Li Y, Sergueev K, Austin S. The segregation of the Escherichia coli origin and terminus of replication. Mol Microbiol 2002; 46:985 - 996
- Wang X, Liu X, Possoz C, Sherratt DJ. The two Escherichia coli chromosome arms locate to separate cell halves. Genes Dev 2006; 20:1727 - 1731
- Reyes-Lamothe R, Possoz C, Danilova O, Sherratt DJ. Independent positioning and action of Escherichia coli replisomes in live cells. Cell 2008; 133:90 - 102
- White MA, Eykelenboom JK, Lopez-Vernaza MA, Wilson E, Leach DR. Non-random segregation of sister chromosomes in Escherichia coli. Nature 2008; 455:1248 - 1250
- Niki H, Yamaichi Y, Hiraga S. Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev 2000; 14:212 - 223
- Bates D, Kleckner N. Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell 2005; 121:899 - 911
- Buenemann M, Lenz P. A Geometrical model for DNA organization in bacteria. PLoS ONE 2010; 5:13806
- Hardy CD, Cozzarelli NR. A genetic selection for supercoiling mutants of Escherichia coli reveals proteins implicated in chromosome structure. Mol Microbiol 2005; 57:1636 - 1652
- Postow L, Hardy CD, Arsuaga J, Cozzarelli NR. Topological domain structure of the Escherichia coli chromosome. Genes Dev 2004; 18:1766 - 1779
- Jun S, Mulder B. Entropy-driven spatial organization of highly confined polymers: lessons for the bacterial chromosome. Proc Natl Acad Sci USA 2006; 103:12388 - 12393
- Jun S, Wright A. Entropy as the driver of chromosome segregation. Nat Rev Microbiol 2010; 8:600 - 607
- Bowman GR, Comolli LR, Zhu J, Eckart M, Koenig M, Downing KH, et al. A polymeric protein anchors the chromosomal origin/ParB complex at a bacterial cell pole. Cell 2008; 134:945 - 955
- Ebersbach G, Briegel A, Jensen GJ, Jacobs-Wagner C. A self-associating protein critical for chromosome attachment, division and polar organization in Caulobacter. Cell 2008; 134:956 - 968
- Weber SC, Spakowitz AJ, Theriot JA. Bacterial chromosomal loci move subdiffusively through a viscoelastic cytoplasm. Phys Rev Lett 2010; 104:238102