 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pathogenic Leptospira protein LigA and LigB are conserved at the N-terminal sequence. In our earlier report, we have presented the spectral properties of individual Big domain of Lig proteins, and showed that an individual domain binds Ca2+. Here we demonstrate that apart from Ca2+-binding properties, the spectral properties (such as doublet Trp fluorescence) shown by an individual domain are almost retained in the protein with many such domains (which could be easily be called as a multimer of an individual tandem repeat). Presence of Asp and Asn in a stretch of sequence in all tandem repeats points towards the possibility of their involvement in Ca2+-binding.
Leptospira is a causative agent of Leptospirosis and the first step in bacterial pathogenesis is its adherence to the host cell surface preceding infection. Leptospires survive both outside and inside a host cells. It is observed that some of the outer membrane proteins are differentially regulated during Leptospirosis. Two of these outer membrane proteins, LigA and LigB, have been shown to interact with extracellular matrix proteins fibronectin, elastin, tropoelastinsCitation1–Citation6 and thus likely mediate adhesion to host cells. Lig proteins belong to the bacterial immunoglobulin-like (Big) family.Citation7 We have earlier shown that individual Big domains of Lig binds Ca2+ which influence the interaction with fibronectin.Citation8 Recently, we have shown the spectral features and Ca2+-binding properties of individual tandem repeats of ∼9 kDa.Citation9 In this work, we have compared their features with the protein containing seven tandem repeats covering the conserved region of Lig proteins (called LigCon).
LigA and LigB consist of 13 and 12 tandem repeats of about 90 amino acids. Amino-terminal regions of LigA and LigB are conserved and contain seven Bacterial immunoglobulin-like domains. We intended to analyze if the spectral features and ion-binding properties shown by an individual domains are similar to that shown by the protein containing several repeats.
Probing Calcium-Binding by Terbium Luminescence
Luminescent calcium mimic probe terbium was used to determine the Ca2+-binding as well as binding affinity of LigCon. Terbium chloride was added to the protein solution and excited at 285 nm. Tb3+ bound to LigCon as indicated by two peaks at 491 and 547 nm which are known to be due to energy transfer from the excited Trp and Tyr (). Interestingly, Trp fluorescence showed a marginal increase of 5% before decreasing to 80% of the maximum with a blue shift of about 10 nm to 340 nm. When Ca2+ was added to the terbium-saturated LigCon, it decreased the terbium fluorescence at 547 nm, while Trp fluorescence increased by 47% over the original start value after calcium addition.
Fluorescence intensities at 547 nm were corrected for dilution and dissociation constant of the protein for terbium were calculated by non-linear curve fitting to the equationCitation10 using the program Microcal Origin 6.0.
Where F, Fmax and Fmin represent the fluorescence intensity at a given point, at saturation and without terbium respectively. Tbf is the free terbium concentration at a given point, which was calculated using the following equation:
Where P is the total protein concentration. The dissociation constant, KD, of LigCon for terbium was in the range of 80 µM.
Far- and Near-UV CD Spectra and Ca2+-Induced Differential Changes in LigCon
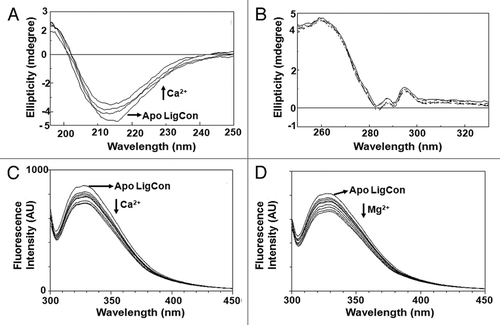
To determine the effect of Ca2+ on secondary and tertiary structure of LigCon, we recorded CD spectra of LigCon in the presence and absence of Ca2+ in far- and near-UV CD spectroscopy. In far-UV CD spectrum, positive and negative peaks at 196 and 216–217 nm, respectively indicates that LigCon is largely in β-sheet conformation (). Outputs of secondary structure fractions from CD spectra of LigCon using CDPro as well as by CDNN programs were more or less similar. Accordingly, apo form of LigCon has 22.6% α-helix, 25.2% β-sheet (antiparallel + parallel), 19.2% turns and 40.8% unordered (CD Spectra deconvolution, version 2.1). Titration with Ca2+ induced moderate changes in far-UV CD spectra (). The secondary structural fractions of holo-LigCon were 19.8% helix, 28.3% beta sheet (antiparallel + parallel), 19.9% turns and 44.1% unordered. Mg2+ titration did not cause any significant changes in far-UV CD (data not shown).
Near-UV CD spectra of LigCon are shown in. The bands for corresponding aromatic amino acids were resolved in near-UV CD with two sharp positive bands of Trp at 288 nm and 296 nm attributing for1Lb transitions of Trp. The intensity of the 296 nm band is sharper than that of the 288 nm band, suggesting that some Trp residues are either immobilized or interact with neighboring aromatic residues.Citation11 There is also a major broad band at about 260 nm characteristic of Phe. There was no significant change in the CD spectra suggesting that these cations do not influence the tertiary structure. This suggests that Lig proteins are not a calcium sensor, but possibly playing other roles such as in bacterial virulence.
Steady-State Fluorescence and Conformational Changes of LigCon by Ca2+ and Mg2+
We next compared the fluorescence spectrum with the spectrum of a single repeat (or domain). The reason for this comparison was that the emission spectrum of any single repeat studied so far has a doublet in a blue shifted spectrum. Although LigCon has seven Trp residues, characteristic doublet, as seen in individual Big domain;Citation9 in the emission maxima at 322 and 330 nm was observed () suggesting that the microenvironment of Trp is similar as in an individual repeat. From emission features, Trp seems to be buried, but exact explanation of such emission needs more investigation. When the LigCon was titrated with increasing concentration of Ca2+, the fluorescence intensity was gradually decreased (). At saturation, there was about 20% decrease in Trp fluorescence intensity. Interestingly, upon binding Mg2+, fluorescence intensity of LigCon also decreased in the same manner as shown by Ca2+ (). These results suggest that both Ca2+ and Mg2+ bind to LigCon and influence the Trp fluorescence.
It is clear that individual Big domains of Lig and LigCon binds Ca2+ and Mg2+, it is not possible to predict the nature of Ca2+-binding sites. Based on the amino acid sequence, we have noted a consensus sequence of DNSNKDITSAVTDxSNxDxxSxVT present in each tandem repeat (). Although it is speculative, the presence of three Asp/Asn residues in this stretch could be among those amino acids involved in Ca2+ ligation. Structural studies are required to understand the pattern of Ca2+-coordination in such proteins.
Figures and Tables
Figure 1 Tb3+ binding to LigCon. 10 µM of purified recombinant LigCon was suspended in 50 mM Tris (pH 7.2) and 50 mM KCl and excited at 285 nm. Emission spectrum was recorded from 300 nm to 580 nm. Aliquots of terbium chloride (0 to 2 mM) were added in the protein solution and spectra were recorded. The intensity at 545 nm was plotted against terbium chloride concentrations. Inset shows emission spectra between 450 and 560 nm upon terbium chloride addition. The two peaks at 485 and 545 nm are seen.
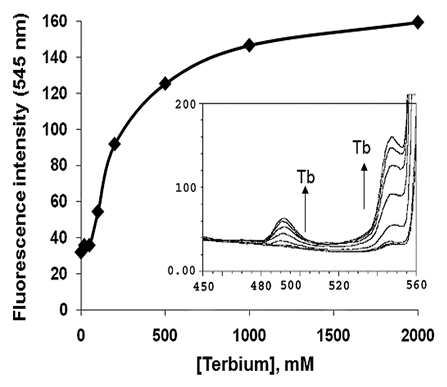
Figure 2 (A) Far-UV CD spectra of LigCon. CD Spectra were recorded using protein concentration of 1.35 mg/ml in a buffer containing 15 mM Tris (pH 7.5), 100 mM KCl and 1 mM DTT. Final calcium concentrations were 0, 100, 200 and 500 µM. Direction of arrows follows the increasing order of calcium concentration. (B) Near-UV CD spectra of LigCon. Protein concentration at 0.86 mg/ml in buffer containing 50 mM Tris (pH 7.5) and 100 mM NaCl was used in a 1 cm path length cuvette. Calcium chloride was added to a final concentration of 0, 100 and 500 µM. Steady-state fluorescence spectra of LigCon and (C) effect of Ca2+ and (D) Mg2+. 10 µM of protein in 20 mM Tris (pH 7.5), 150 mM KCl and 1 mM DTT was excited at 295 nm. Aliquots of calcium chloride or magnesium chloride from respective stock solutions were added until saturation was reached. The figure shows Trp fluorescence in the presence of 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.7, 1 and 2 mM of CaCl2.
Acknowledgements
This work was supported in part by the Biotechnology Research and Development Corporation (BRDC) and DBT as well as DST (Govt. of India). Rajeev Raman is supported by a senior research fellowship from the Council of Scientific and Industrial Research, Government of India.
Addendum to:
References
- Lin YP, Chang YF. A domain of the Leptospira LigB contributes to high affinity binding of fibronectin. Biochem Biophys Res Commun 2007; 362:443 - 448
- Lin YP, Chang YF. The C-terminal variable domain of LigB from Leptospira mediates binding to fibronectin. J Vet Sci 2008; 9:133 - 144
- Choy HA, Kelley MM, Chen TL, Moller AK, Matsunaga J, Haake DA. Physiological osmotic induction ofLeptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect Immun 2007; 75:2441 - 2450
- Lin YP, Greenwood A, Nicholson LK, Sharma Y, McDonough SP, Chang YF. Fibronectin binds to and induces conformational change in a disordered region of Leptospira immunoglobulin-like protein B. J Biol Chem 2009; 284:23547 - 23557
- Lin YP, Lee DW, McDonough SP, Nicholson L, Sharma Y, Chang YF. Repeated domains of Leptospira Immunoglobulin-like proteins interact with elastin and tropoealstin. J Biol Chem 2009; 284:19380 - 19391
- Lin YP, McDonough SP, Sharma Y, Chang YF. The terminal Immunoglobulin-like repeats of LigA and LigB of Leptospira enhance their binding to gelatin binding domain of fibronectin and host cells. PLoS One 2010; 5:11301
- Palaniappan RU, Chang YF, Jusuf SS, Artiushin S, Timoney JF, McDonough SP, et al. Cloning and molecular characterization of an immunogenic LigA ofLeptospira interrogans. Infect Immun 2002; 70:5924 - 5930
- Lin YP, Raman R, Sharma Y, Chang YF. Calcium binds to Leptospiral immunoglobulin-like protein, LigB and modulates fibronectin binding. J Biol Chem 2008; 283:25140 - 25149
- Raman R, Rajanikanth V, Palaniappan RU, Lin YP, He H, McDonough SP, et al. Big domains are novel Ca2+-binding modules: evidences from big domains of Leptospira immunoglobulin-like (Lig) proteins. PLoS ONE 2010; 5:14377
- Drake SK, Falke JJ. Kinetic tuning of the EF-hand calcium binding motif: the gateway residue independently adjusts (i) barrier height and (ii) equilibrium. Biochemistry 1996; 35:1753 - 1760
- Woody RW, Dunker AK. Fasman GD. In circular dichroism and the conformational analysis of biomolecules 1996; New York Plenum 109 - 157
