Abstract
Exposure of phagocytes to non-spherical particles has provided evidence for multiple actions of the actin system in force generation. For the uptake of long cylindrical particles, a “motile actin clamp” mechanism is proposed. When a phagocyte is engaged with an hourglass-shaped particle, it exerts contractile activity alternatively at the far end of the particle or at its concave region. Phagocytes can switch within seconds between these different strategies of taking up a particle. This response switching is based on reprogramming the pattern of actin polymerization and depolymerization. The choice between different strategies of interaction with a particle increases the probability of engulfing the entire particle or at least a portion of it. Finally, a switch to actin disassembly enables a phagocyte to release a particle that turns out to be too big to be enclosed.
Responses to a Complex Signal: Stochastic Transitions between Multiple States
Phagocytosis requires accommodation of the actin system to various shapes of a target particle and in particular detection of the end of the particle where the phagocytic cup can close by membrane fusion. To explore the regulatory capacities of the actin system in phagocytosis, we have exposed professional phagocytes, the amoeboid cells of Dictyostelium, to particles of various shapes (). Specifically, we used yeast mutants that formed either hourglass-shaped particles consisting of two convex lobes connected by a concave neckCitation1 or long cylindrical particles.Citation2 The bi-lobed particles revealed that a phagocyte has at least three options to deal with a particle of complex shape: (1) to spread over both the convex and concave regions of the particle surface until the phagocytic cup closes at the actual end of the particle; (2) to stop at the concave neck and apply force there in an attempt to sever the particle and take up one of its two lobes; and (3) to disengage and release the particle if attempts (1 and 2) should fail. This variability of the response is possible because actin polymerization is positively and negatively regulated along the entire phagocytic cup.
Typically, signal transduction systems are designed so that a signal is translated into an unequivocal response. For instance, in the chemotactic response of a eukaryotic cell the external gradient is reliably translated into a cellular pattern of activities that guide the cell toward the source of chemoattractant. This unique signal-response relationship is not necessarily the optimal mode of responding to a complex signal, such as a large particle of irregular shape. Therefore, mechanisms have evolved that allow the response to switch between two or more modes in order to increase the probability of success. In this manner, a phagocytosing cell benefits from accessing multiple activities elicited by a single signal, the complex shape of an attaching particle. If severing at a concave region is not successful, the phagocytic cup may further expand to engulf the entire entity, or the actin network surrounding the particle may disassemble, resulting in the particle's release. There is no fixed temporal order of progression from one mode to the other, suggesting that the transitions between different states of the response system are based on stochastic switches.
Multitude of Forces Involved in Particle Uptake
The regulation of actin assembly enables a phagocyte to modify local force generation within seconds in order to spread along a particle, to pause or to retract the cup. Forces involved in phagocytosis have previously been determined by measuring the increase of cortical tension over the entire cell surface during the uptake of a large particle.Citation3 The uptake of a particle of any shape has to act against cortical tension, which increases abruptly when the membrane area consumed for phagocytic cup formation exceeds a certain limit.Citation4 The global measurement of cortical tension yields the force that the actin-based uptake mechanism needs to generate in order to recruit the membrane area required for the phagosomal membrane to envelop the particle. This membrane area is internalized and finally separated from the plasma membrane that surrounds the cell. However, this type of measurement does not comprise the full set of forces generated in phagocytosis. For instance, the contraction and severing of the neck region of a bi-lobed particle will cause little change in the cell surface area. Therefore, the contribution of this force to cortical tension will be negligible, although the local force applied to the particle appears to be strong. Moreover, the ability of a phagocyte to simultaneously take up one particle while releasing another indicates that force is regulated independently on each individual particle, rather than through global activation of the phagocyte.Citation1
The contractile activities considered so far tend to close an orifice, either at the rim of a phagocytic cup or around the concave neck region of a particle. It may be asked whether a long cylindrical particle is taken up by the same mechanism. A particle of that shape has to be pulled into the cell with no possibility for the phagocytic cup to contract before it reaches the end of the particle (). The energy of phagocyte-to-particle adhesion is certainly not sufficient for uptake, since as soon as the actin coat surrounding the phagosome membrane begins to disassemble, the particle is expelled.Citation2 I hypothesize that a cylindrical particle is taken up by a “motile clamp” mechanism, with actin rings encircling the particle. These rings are supposed to have two functions: to act as clamps for preventing the particle from escaping, and to move the particle into the cell by a mechanism similar to that of cell migration on a planar surface.Citation2 Most likely motor proteins of the myosin-I family are involved in elongating the phagocytic cup and carrying the particle into the cell.
Perspectives
The yeast used in our work are optimally suited to investigate the interaction of phagocytes with particles similar to those present in their natural habitat. However, these particles are not sufficiently well-defined to allow measurement of forces and to analyze the effects of mutations that alter actin organization in the phagocytes or eliminate the activity of individual myosin isoforms. Therefore, further analysis of actin responses to curvature-dependent signals should make use of artificial particles of defined shape such as those produced by Champion and Mitragotri,Citation5 and the measurement of actin-based force generation on individual phagosomes during consecutive stages of their uptake might employ synthetic polymers of defined mechanical properties as introduced by Beningo and Wang.Citation6
Figures and Tables
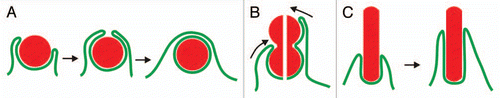
Figure 1 Interaction of a phagocyte with particles of different shapes. The diagram illustrates the uptake of three types of particles: spherical ones with a convex surface of constant curvature (A), bi-lobed particles with a concave neck (B) and long cylindrical particles with parallel contour aspect (C). (A) the internalization of a sphere occurs in two phases. First, the phagocytic cup needs to expand without the application of contractile forces, since otherwise the particle would be repelled (left part). Second, the cup has to contract around the particle (middle) until it can close on top of the particle for separation of the phagosome from the cell surface (right). (B) a bi-lobed particle imposes a conflict on the phagocyte: should the concave neck be taken as the end of the particle (left possibility in the split image) or should the cup continue to progress in search of a more distal end (right possibility). (C) a rod-shaped particle will be drawn into the phagocyte by gliding movement of the phagocytic cup along the particle's constant perimeter.
Acknowledgements
I would like to acknowledge support of the Max Planck Society, and to thank Margaret Clarke, the first author of the paper to which this Addendum applies, for comments.
Addendum to:
References
- Clarke M, Engel U, Giorgione J, Müller-Taubenberger A, Prassler J, Veltman D, et al. Curvature recognition and force generation in phagocytosis. BMC Biol 2010; 8:154
- Gerisch G, Ecke M, Schroth-Diez B, Gerwig S, Engel U, Maddera L, et al. Self-organizing actin waves as planar phagocytic cup structures. Cell Adh Migr 2009; 3:373 - 382
- Evans E, Leung A, Zhelev D. Synchrony of cell spreading and contraction force as phagocytes engulf large pathogens. J Cell Biol 1993; 122:1295 - 1300
- Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: behavior of the cortical tension. J Cell Sci 2005; 118:1789 - 1797
- Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA 2006; 103:4930 - 4934
- Beningo KA, Wang YL. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci 2002; 115:849 - 856