Abstract
Mitochondria are highly dynamic organelles that are central to several cellular processes, the most prominent being energy production. Several reports have shown that pathogens target mitochondria in various ways to interfere with apoptosis, but to our knowledge only one study has specifically addressed the effects of infection on mitochondrial dynamics. We focused on this aspect during infection with the intracellular pathogen L. monocytogenes and could recently show that this bacterium profoundly alters mitochondrial dynamics, causing transient fragmentation of the mitochondrial network. This mitochondrial fragmentation occurs early during infection and is specific to pathogenic L. monocytogenes, as it is not observed with other intracellular pathogens. The relevance of mitochondrial dynamics for L. monocytogenes infection is highlighted by the finding that siRNA-mediated inhibition of mitochondrial fusion or fission decreases or increases the efficiency of L. monocytogenes infection, respectively. The main bacterial factor responsible for mitochondrial network disruption was identified as the secreted pore-forming toxin listeriolysin O, which also appeared to impair mitochondrial function. Our work suggests that in order to establish an efficient infection, L. monocytogenes interferes with cellular physiology at early timepoints by transient disruption of mitochondrial dynamics and function
Mitochondria are crucial organelles. They provide most of the cellular ATP, and are also increasingly recognized as signaling platforms in biological processes as diverse as apoptosisCitation1,Citation2 and innate immune signaling.Citation3 Mitochondria hence represent sites where signaling and energy-producing activity are integrated.Citation4 In addition, under different physiological conditions, changes in mitochondrial activity influence mitochondrial morphology and vice versa.Citation5 Mitochondrial fission is essential for proper distribution of mitochondria (and thereby of energy supply) within the cell,Citation6,Citation7 for the elimination of nonfunctional mitochondria,Citation8 for mitochondrial inheritance during mitosisCitation9 and for embryonic development.Citation10–Citation12
Due to their key role in cell survival, mitochondria represent attractive targets for pathogens. Interfering with mitochondria can aim at preserving the bacterial replication niche by inhibiting death of the host cell, or at inducing death of immune cells that counteract pathogen dissemination.Citation13 Several pathogens, including both viruses and bacteria, have been shown to target mitochondria in order to interfere with the host cell apoptotic machinery (reviewed in ref. Citation14 and Citation15). Apoptotic cell death eventually entails fragmentation of the mitochondrial network,Citation16 and bacteria that induce apoptosis can thus lead to mitochondrial network fragmentation. On the other hand, evidence is accumulating that induction of mitochondrial fragmentation is not sufficient to cause cell death.Citation17,Citation18
Only few data are available on the effects of intracellular pathogens on host cell mitochondrial dynamics and function under conditions where apoptosis is not induced. This prompted us to specifically analyze the effect on host cell mitochondria upon infection with an invasive bacterial pathogen, Listeria monocytogenes. In our study, we show for the first time that infection with L. monocytogenes alters the morphology of host cell mitochondria, inducing fast and drastic fragmentation of the mitochondrial network. We have identified the secreted bacterial toxin listeriolysin O (LLO) as the main factor causing mitochondrial fragmentation via the induction of calcium fluxes at early time-points of infection. LLO therefore differs from bacterial effectors that are directly targeted to mitochondria and insert into the outer membrane or effectors that are imported into the mitochondrial matrix (reviewed in ref. Citation15 and Citation19). The rapid fission kinetics observed in the case of Listeria infection leads us to postulate that local production of small amounts of LLO before or during bacterial entry is sufficient to induce such fragmentation. In this context, LLO affects also bystander cells, therefore acting as a classical diffusible toxin. Interestingly, previous studies have shown that not only inactivation of extracellular LLO with a monoclonal antibody (A4–8) renders wild type Listeria avirulent,Citation20 but also that a non-secreted, surface-associated version of LLO causes a decrease in in vivo infectivity and virulence despite a normal in vitro phenotype.Citation21 These data support the notion that several effects of LLO occur well before bacterial entry and depend on its ability to form pores on the plasma membrane.
While we could not detect rapid mitochondrial fragmentation upon infection with several other intracellular bacterial pathogens, e.g., Shigella and Salmonella, recombinant pore-forming toxins of the same family as LLO appeared to have a similar effect as LLO on mitochondria. Some of these pore-forming toxins, e.g., S. pneumoniae pneumolysin (PLY) and S. pyogenes streptolysin O (SLO) have been shown to activate the apoptotic cascade by damaging mitochondria.Citation22,Citation23 However, as we showed, Listeria induces mitochondrial fragmentation without classical apoptosis, suggesting that LLO action differs from that of pore-forming toxins produced by extracellular bacteria. Interestingly, the only other study explicitly analyzing mitochondrial dynamics upon infection showed that Helicobacter pylori causes cell death-associated mitochondrial fragmentation.Citation24
The molecular mechanism that mediates the LLO-induced mitochondrial fission is still unclear. Due to the rapid kinetics of the process, we speculate that the fission machinery is activated by a calcium-dependent mechanism. We currently cannot exclude that in addition the fusion machinery is blocked due to the LLO-induced decrease in mitochondrial membrane potential.Citation5
Our work links mitochondrial dynamics with bacterial infection using the intracellular pathogen Listeria as a model. Two elegant reports have recently linked mitochondrial dynamics to innate immunity in the context of viral infection: mitochondrial morphology, and in particular mitofusins were shown to regulate mitochondrial antiviral signaling protein (MAVS).Citation25,Citation26 It is possible that bacterial pathogens also take advantage of the connection between innate immunity and mitochondria and Listeria is certainly a prime candidate to address this hypothesis. Interestingly, MAVS signaling was recently shown to depend on the energetic status of mitochondria.Citation27 This result confirms the intuitive notion that cellular bioenergetics is critical for correct functioning of defense mechanisms. Indeed, mitochondrially diseased cells are more susceptible to Legionella infection.Citation28 Our work supports the existence of a connection between bioenergetics and bacterial infection. Concomitant to mitochondrial fragmentation, Listeria infection induces a decrease in the mitochondrial membrane potential and in respiration, which possibly contributes to the bioenergetic crisis that is also reflected by a significant drop in intracellular ATP levels (). We speculate that these processes are beneficial for the initial establishment of infection, particularly because evidence is accumulating that beyond providing energy, metabolites play important signaling functions.Citation29 Detailed analyses are now required to shed light onto which pathways mediate the link between host cell bioenergetics and Listeria invasion.
Figures and Tables
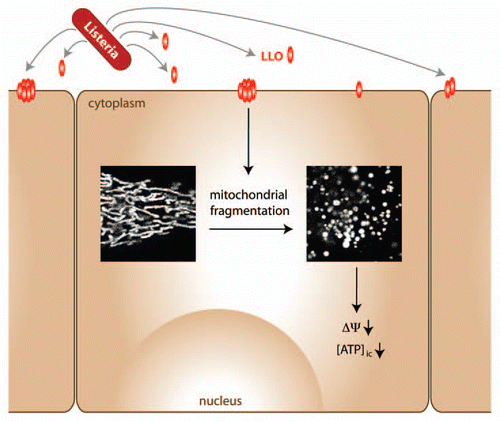
Figure 1 Model depicting the effect of Listeria infection on host cells. Early during Listeria infection, secreted LLO inserts into the plasma membrane of host cells (which can also be bystander cells), causing fragmentation of the mitochondrial network along with a decrease in mitochondrial membrane potential and intracellular ATP levels. Cellular signaling pathways that remain to be identified may sense changes in mitochondrial dynamics and bioenergetics to impact on infection efficiency.
Addendum to:
References
- Wasilewski M, Scorrano L. The changing shape of mitochondrial apoptosis. Trends Endocrinol Metab 2009; 20:287 - 294
- Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet 2009; 43:95 - 118
- Arnoult D, Carneiro L, Tattoli I, Girardin SE. The role of mitochondria in cellular defense against microbial infection. Semin Immunol 2009; 21:223 - 232
- Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta 2009; 1793:154 - 170
- Sauvanet C, Duvezin-Caubet S, di Rago JP, Rojo M. Energetic requirements and bioenergetic modulation of mitochondrial morphology and dynamics. Semin Cell Dev Biol 2009; 21:558 - 565
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci 2005; 118:5411 - 5419
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004; 119:873 - 887
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008; 183:795 - 803
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 2007; 282:11521 - 11529
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 2009; 11:958 - 966
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell 1999; 4:815 - 826
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol 2009; 186:805 - 816
- Bohme L, Rudel T. Host cell death machinery as a target for bacterial pathogens. Microbes Infect 2009; 11:1063 - 1070
- Galluzzi L, Brenner C, Morselli E, Touat Z, Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog 2008; 4:1000018
- Rudel T, Kepp O, Kozjak-Pavlovic V. Interactions between bacterial pathogens and mitochondrial cell death pathways. Nat Rev Microbiol 2010; 8:693 - 705
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynaminrelated protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 2001; 1:515 - 525
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 2008; 14:193 - 204
- Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell 2009; 36:355 - 363
- Kozjak-Pavlovic V, Ross K, Rudel T. Import of bacterial pathogenicity factors into mitochondria. Curr Opin Microbiol 2008; 11:9 - 14
- Edelson BT, Cossart P, Unanue ER. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J Immunol 1999; 163:4087 - 4090
- Carrero JA, Calderon B, Vivanco-Cid H, Unanue ER. Recombinant Listeria monocytogenes expressing a cell wall-associated listeriolysin O is weakly virulent but immunogenic. Infect Immun 2009; 77:4371 - 4382
- Timmer AM, Timmer JC, Pence MA, Hsu LC, Ghochani M, Frey TG, et al. Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J Biol Chem 2009; 284:862 - 871
- Braun JS, Hoffmann O, Schickhaus M, Freyer D, Dagand E, Bermpohl D, et al. Pneumolysin causes neuronal cell death through mitochondrial damage. Infect Immun 2007; 75:4245 - 4254
- Ashktorab H, Frank S, Khaled AR, Durum SK, Kifle B, Smoot DT. Bax translocation and mitochondrial fragmentation induced by Helicobacter pylori. Gut 2004; 53:805 - 813
- Yasukawa K, Oshiumi H, Takeda M, Ishihara N, Yanagi Y, Seya T, et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal 2009; 2:47
- Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep 2009; 11:133 - 138
- Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal 2011; 4:7
- Francione L, Smith PK, Accari SL, Taylor PE, Bokko PB, Bozzaro S, et al. Legionella pneumophila multiplication is enhanced by chronic AMPK signalling in mitochondrially diseased Dictyostelium cells. Dis Model Mech 2009; 2:479 - 489
- Laporte D, Lebaudy A, Sahin A, Pinson B, Ceschin J, Daignan-Fornier B, et al. Metabolic status rather than cell cycle signals control quiescence entry and exit. J Cell Biol 2011; 193:1 - 9