Abstract
The eukaryotic plasma membrane is organized into distinct domains that contribute to its function. One new type of plasma membrane domain was identified by studies on the Sur7 protein, which was discovered in the yeast S. cerevisiae to localize into stable punctate patches known as MCC or eisosomes. Sur7 shares similarities with Claudin proteins that form tight junction domains in animal cells, suggesting common roles for these tetraspanning membrane proteins. Recent analysis of C. albicans revealed broad new roles for Sur7; a sur7Δ mutant mislocalized septins and actin and was defective in morphogenesis. Strikingly, cell wall synthesis was very abnormal, including long projections of chitin-rich cell wall into the cytoplasm. Some phenotypes of the sur7Δ mutant are similar to the effects of inhibiting cell wall β-glucan synthesis. This suggests that the abnormal cell wall structures are related to the increased chitin synthesis commonly seen under cell wall stress conditions, which could be mediated in part by the altered septin localization. Altogether, these results identify new roles for Sur7 and MCC/eisosomes in plasma membrane organization and coordination of the different aspects of cell wall synthesis.
The plasma membrane is more than just an important barrier; it is a dynamic organelle that mediates a wide array of cellular functions. Eukaryotic plasma membranes are thought to be composed of distinct subdomains, including lipid rafts and protein-organized domains.Citation1–Citation3 In the case of the budding yeast Saccharomyces cerevisiae, the plasma membrane includes at least two types of domains.Citation4,Citation5 One domain termed MCP contains proteins that readily diffuse, such as the plasma membrane ATPase Pma1. Another domain appears as 300 nm-sized immobile patches. This latter domain was termed MCC for “Membrane Compartment occupied by Can1”, since it contains the Can1 arginine permease.Citation4,Citation5 These punctate domains have also been termed “eisosomes” because of their contribution to endocytosis.Citation6
What Are MCC/Eisosomes and How do they Form?
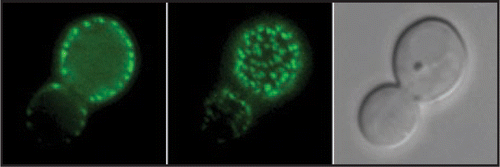
Analysis of the S. cerevisiae Sur7 protein first revealed its presence in immobile punctate patches in the plasma membraneCitation7 and similar results were observed in C. albicansCitation8 (). It is not understood why Sur7 and MCC/eisosomes are immobile. The restricted mobility is not dependent on actin, microtubules, or the cell wall.Citation5 MCC/eisosomes are thought to be rich in sterols,Citation9 and contain additional proteins whose identification is helping to define the role of these domains.
The formation of MCC/eisosomes is dependent on sphingolipid production and the sphingolipid-responsive Pkh1/2 protein kinases.Citation10,Citation11 Sur7 does not play an essential role in the ability of the other MCC/eisosomes proteins to form punctate patches. However, Sur7 contains four transmembrane domains, suggesting it could anchor cytoplasmic proteins. Sur7 also shares a conserved motif in extracellular loop 1 with Claudin proteins that form tight junctions in animal cells, suggesting a common role for these tetraspanning membrane proteins in specialized membrane domains.Citation8,Citation12
What Roles do MCC/Eisosomes Play in Plasma Membrane Function?
One potential role is to organize proteins with similar proton flux activities. This was suggested by the discovery that proton symporters for arginine, uracil and tryptophan localize to MCC/eisosomes in S. cerevisiae.Citation4,Citation5,Citation9 Segregation of the inward proton flux of the symporters away from the outward proton pumping activity of the plasma membrane H+ATPase Pma1 may therefore have important physiological significance.Citation4,Citation9 The proton symporters are distinct from Sur7 in that their presence in MCC/eisosomes is dependent on the membrane potential.Citation9
A role for MCC/eisosomes in endocytosis was first suggested because SUR7 overexpression suppressed the growth defects of an rvs167 endocytosis mutant in S. cerevisiae.Citation13 Subsequently, other proteins needed for efficient endocytosis were also localized to MCC/eisosomes, including Pil1, Lsp1 and Pkh1/2.Citation6,Citation10,Citation11 Sites of active endocytosis were localized to MCC/eisosomes in one study, but it is not clear how they relate to the sites of endocytosis mediated by actin patches in the plasma membrane, since these regions appear to be distinct.Citation6,Citation7
Detection of the Pkh1/2 protein kinases in the eisosome/MCC patches has also broadened the roles of these domains. The Pkh1/2 protein kinases associate with and phosphorylate Pil1 and Lsp1 in MCC/eisosomesCitation10,Citation11 and influence other processes in addition to endocytosis including, cell wall integrity, actin localization and response to heat stress.Citation14,Citation15 The Pkh1/2 protein kinases also mediate the effects of sphingolipids on the assembly and disassembly of eisosomes.Citation10,Citation11
A Role for Ca-Sur7 in Cell Wall Synthesis in C. albicans
The function of Sur7 in S. cerevisiae is not clear since deletion of Sc-SUR7 caused only minor effects.Citation7 Perhaps this is due to genetic redundancy.Citation7,Citation8 In contrast, a C. albicans sur7Δ mutant displayed obvious defects in endocytosis and morphogenesis.Citation8 Septins and actin were mislocalized, and cell wall synthesis was very abnormal, including long projections of cell wall into the cytoplasm. Several phenotypes of the sur7Δ mutant are similar to the effects of inhibiting β-glucan synthase, suggesting that the abnormal cell wall synthesis is related to activation of chitin synthase seen under stress conditions.Citation8 Since septins are involved in recruiting chitin synthase to the sites of cytokinesis, the altered septin localization in the sur7Δ mutant is implicated in promoting this aberrant cell wall synthesis. Thus, altered septin localization in the sur7Δ mutant may represent part of a stress-activated induction of chitin synthesis that acts as a compensatory response to disruption of normal cell wall growth. These results expand the roles of MCC/eisosomes by demonstrating that Sur7 is needed for proper plasma membrane organization and cell wall synthesis.
Figures and Tables
Figure 1 Localization of Sur7-GFP to punctate patches in the plasma membrane. C. albicans strain YJA15 carrying a SUR7-GFP fusion gene was analyzed by fluorescence microscopy. An image of the medial focal plane is on the left, the top of the cell is in the middle, and a light microscope image of the same cell is shown on the right.
Acknowledgements
This work was supported by research grant RO1 AI47837 from the National Institutes of Health that was awarded to James B. Konopka.
Addendum to:
References
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 2000; 275:17221 - 17224
- Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, et al. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol 2003; 5:626 - 632
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005; 6:801 - 811
- Malinska K, Malinsky J, Opekarova M, Tanner W. Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol Biol Cell 2003; 14:4427 - 4436
- Malinska K, Malinsky J, Opekarova M, Tanner W. Distribution of Can1p into stable domains reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells. J Cell Sci 2004; 117:6031 - 6641
- Walther TC, Brickner JH, Aguilar PS, Bernales S, Pantoja C, Walter P. Eisosomes mark static sites of endocytosis. Nature 2006; 439:998 - 1003
- Young ME, Karpova TS, Brugger B, Moschenross DM, Wang GK, Schneiter R, et al. The Sur7p family defines novel cortical domains in Saccharomyces cerevisiae, affects sphingolipid metabolism, and is involved in sporulation. Mol Cell Biol 2002; 22:927 - 934
- Alvarez FJ, Douglas LM, Rosebrock A, Konopka JB. The Sur7 protein regulates plasma membrane organization and prevents intracellular cell wall growth in Candida albicans. Mol Biol Cell 2008; In press
- Grossmann G, Opekarova M, Malinsky J, Weig-Meckl I, Tanner W. Membrane potential governs lateral segregation of plasma membrane proteins and lipids in yeast. EMBO J 2007; 26:1 - 8
- Walther TC, Aguilar PS, Frohlich F, Chu F, Moreira K, Burlingame AL, et al. Pkh-kinases control eisosome assembly and organization. EMBO J 2007; 26:4946 - 4955
- Luo G, Gruhler A, Liu Y, Jensen ON, Dickson RC. The sphingolipid long-chain base-Pkh1/2-Ypk1/2 signaling pathway regulates eisosome assembly and turnover. J Biol Chem 2008; 283:10433 - 10444
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol 2006; 16:181 - 188
- Sivadon P, Peypouquet MF, Doignon F, Aigle M, Crouzet M. Cloning of the multicopy suppressor gene SUR7: evidence for a functional relationship between the yeast actin-binding protein Rvs167 and a putative membranous protein. Yeast 1997; 13:747 - 761
- Dickson RC. Sphingolipids. New insights into sphingolipid metabolism and function in budding yeast. J Lipid Res 2008; 49:909 - 921
- Dickson RC, Sumanasekera C, Lester RL. Functions and metabolism of sphingolipids in Saccharomyces cerevisiae. Prog Lipid Res 2006; 45:447 - 465