Abstract
In plant-pathogen interactions, pathogens aim to overcome host defence responses while plants employ a battery of responses to limit pathogen growth and thus disease. In this “arms race” between hosts and pathogens, horizontal gene transfer is a potent source of ‘pathogenic innovation’ for viruses and bacteria. However, bacteria rarely acquire ‘eukaryotic-like’ genes from their hosts, and where they appear to, evidence for a role of the acquired genes remains outstanding. We have recently reported experimental evidence that the citrus canker causing pathogen Xanthomonas axonopodis pv. citri contains a plant natriuretic peptide-like gene (XacPNP) that encodes a protein that modulates host homeostasis to its advantage. We argue that Xanthomonas PNP has been acquired in an ancient horizontal gene transfer, and given that plant and bacterial PNPs trigger a number of similar physiological responses, we make a case of molecular mimicry. Released XacPNP mimics host PNP and results in a suppressed host response, “improved” host tissue health and consequently better pathogen survival in the lesions. Finally, we propose that Xanthomonas axonopodis pv. citri host interactions can serve as model system to study the role of host homeostasis in plant defence against biotrophic pathogens.
There are many phytopathogenic microbes in Phytophthora genus, which usually cause annual losses worldwide. In the field, fungus-like mycelia of Phytophthora differentiate to form multinucleate asexual sporangia, which could germinate to infect host plant or cleave cytoplasm to form and release uninucleate and biflagellated zoospores.Citation1 Motile zoospores swim toward host plant through sensing chemical signals secreted from roots of hosts, and begin new infection.Citation2 Consequently, asexual development including sporangia formation, zoospore motility and chemotaxis to special chemical signals determine the ability of dispersal and host invasion of Phytophthora. Recently, we have proven that Gα mediated signal transduction pathway controlled P. sojae zoospore chemotaxis to soybean isoflavone.Citation3 Silencing of PsGPA1 gene, which encodes Gα, caused upregulation of calcium binding proteins including calmodulin and protein kinase. Further more, we found some other changes of phenotypes caused by PsGPA1 gene silencing and downstream targets regulated by Gα. In this addendum, we described the function of Gα in helping zoospores to find penetration sites, effectors whose transcription were influenced by Gα, and different functions of Gα between P. infestans and P. sojae.
Gα Help P. sojae Zoospore to Find Not Only Host, but also Penetration Site
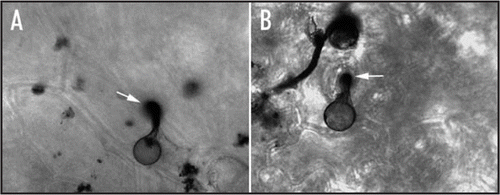
Gα participates the signal transduction pathway, which controls P. sojae zoospore swimming to soybean isoflavone daidzein and some amino acids. In addition, it could help P. sojae zoospore to find best penetration site. We dropped zoospore suspensions of P. sojae Gα silenced mutant and wild-type strain onto the epidermis of onion. After 30 min in 25°C, zoospore encystment on epidermis of onion was observed under microscope. As show in , most zoospores of wild-type strain encysted on the gap of two cells, and aggregated together, while zoospores of Gα silenced mutant encysted randomly on the surface of epidermis without aggregation. However, the same phenomenon was not observed on epidermis of soybean. Zoospores of P. sojae wild-type strain can penetrate soybean cell from either intercellular gap or normal cell wall (), which may be due to the different structure or components of plant cells. This result indicates that Gα has putative function on helping zoospore to find the best site of host for infection.
Gα and RGS Protein
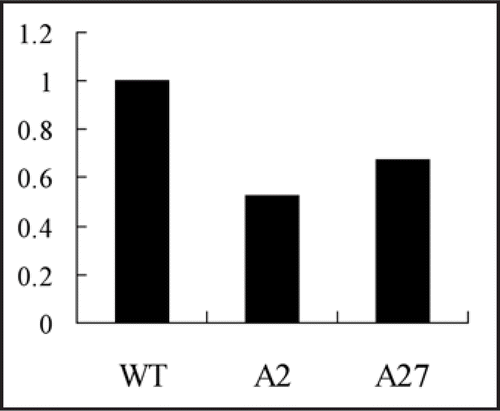
Gα transmits extracellular signals from G protein coupled receptors, which combined ligands, to downstream targets including adenylyl cyclase, phospholipase and ion channels.Citation4 It has been proven that silencing of Gα can cause upregulation of some calcium binding proteins.Citation3 In addition, there are also some genes expressed downregulated following silencing of Gα. For example, expression of a gene-coding regulator of G protein signal (RGS), named PsRGS6 was analyzed in Gα silenced mutant and wild-type strain. The result showed that, PsRGS6 was expressed lower in Gα silenced mutant than that in wild-type strain (). RGS proteins have GTPase activity, which could hydrolyze GTP to GDP. That would allow Gα-GTP transforms to Gα-GDP, which reassociates with Gβγ dimmer and inhibit Gα mediated signal transduction.Citation5 Based on our results, PsRGS6 may function as an inhibitor of G protein signal, whose transcription is regulated by Gα.
Different Function of Gα Between P. infestans and P. sojae
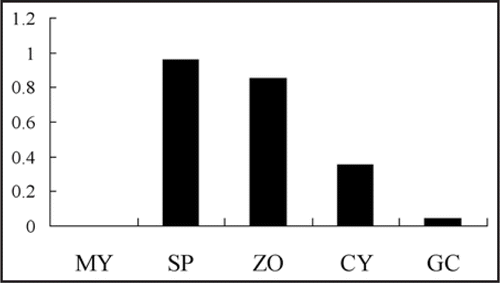
P. sojae and P. infestans are two representative Phytophthora species with differences in development and host range. P. infestans is a air-borne species, sporangia of which can be separated from sporangiophores, and usually spread by wind or water to new potential sites of infection. Sporangial cleavage of P. infestans needs cool and moist conditions. In contrast, P. sojae is a soil-borne species, sporangia of which cannot be separated from sporangiophores, and sporangial cleavage does not need cool condition. The most important difference is that P. sojae zoospore can be attracted not only by some amino acids, which is the same as that of P. infestans, but also by isoflavones secreted from soybean roots, which may be partly determined by host range. These differences may be the reason for Gα mutants of P. infestans and P. sojae have some different phenotypes. There is only one copy of Gα subunit gene in all the sequenced Phytophthora genome. From the results of Northern Blot and RT-PCR, Gα is not expressed in nutrient mycelium, while it is expressed highest in sporangia or sporulating mycelia.Citation3,Citation6 However, there are still some different expression patterns of Gα in asexual development between P. sojae and P. infestans. Zoospore has to encyst to host surface before penetration. Gα are expressed in the same levels between zoospore and cyst in P. infestans.Citation6 However, in P. sojae, it is expressed higher in zoospore than that in cyst (). So zoospore of Gα silenced mutant encysts very quickly than that of wild-type strain,Citation3 while P. infestans Gα silenced mutant did not have the same phenotype in zoospore encystment. Gα mutant of P. sojae and P. infestans reduced their pathogenisity by different mechanisms. P. sojae zoospore reduced its ability of cyst germination, while P. infestans reduced its ability of appressorium formation. For P. infestans, the expression level of Gα in sporangium is much higher than that in zoospore, while for P. sojae, the expression levels in those two stages are similar. The reason may be in different functions of Gα in P. sojae and P. infestans. This indicates that G protein signal pathway in different Phytophthora spp. may participate in different signal transduction pathways.
Conclusions and Future Directions
The function of Gα in sporangial cleavage, zoospore motility and pathogenesis of Phytophthora has been found. However, there are no evidence revealed that Gα participates sexual development of Phytophthora. We supposed that, hormone signal could be transmitted independently from Gα mediated signal transduction pathway. There are 24 GPCRs in P. sojae genome, 12 of which are fused to a PIPK domain which are similar to Dictyostelium RpkA.Citation7 These GPCR-PIPKs may transmit extracellular signals to cells which trigger phosphoinositide second messenger synthesis, and activate downstream signaling pathways which control sexual development of P. sojae. Although transcription of some putative downstream targets of G protein such as calcium binding proteins and RGS protein were found to be regulated by Gα, more effectors binding with Gα and regulated by Gα need to be found. The G protein involved signal transduction mechanisms also need to be deeply analyzed for disease control.
Figures and Tables
Figure 1 Zoospore encystment on the epidermis of onion. Zoospore suspension of wild-type strain and Gα silenced mutant were dropped on the surface of epidermis of onion, and incubated in 25°C. After 30 min, zoospore encystment was analyzed under microscope. (A) Zoospore encystment of wild-type strain on epidermis of onion. (B) Zoospore encystment of Gα silenced mutant on epidermis of onion.
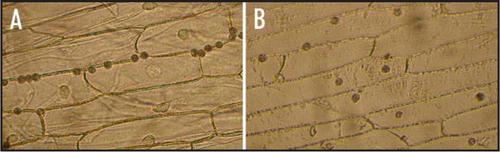
Figure 2 Soybean leaves infected by P. sojae zoospores. (A) P. sojae germ tube penetrated through intercellular space of soybean leaf. (B) P. sojae germ tube penetrated through stoma of soybean leaf. The arrows point to the penetrating site of germ tubes.
Addendum to:
References
- Erwin DC, Ribeiro OK. Phytophthora Diseases Worldwide: American Phytopathological Society 1996; St. Paul, MN, USA
- Tyler BM. Phytophthora sojae: root rot pathogen of soybean and model oomycete. Molec Plant Pathol 2007; 8:1 - 8
- Hua C, Wang Y, Zheng X, Dou D, Zhang Z, Govers F, Wang Y. A Phytophthora sojae G Protein {alpha} Subunit is Involved In Chemotaxis To Soybean Isoflavones. Eukaryot Cell 2008; In Press
- Malbon CC. G proteins in development. Nat Rev Mol Cell Biol 2005; 6:689 - 701
- Wang Y, Ho G, Zhang JJ, Nieuwenhuijsen B, Edris W, Chanda PK, et al. Regulator of G protein signaling Z1 (RGSZ1) interacts with Galphai subunits and regulates Galphai-mediated cell signaling. J Biol Chem 2002; 277:48325 - 48332
- Maria Laxalt A, Latijnhouwers M, van Hulten M, Govers F. Differential expression of G protein alpha and beta subunit genes during development of Phytophthora infestans. Fungal Genet Biol 2002; 36:137 - 146
- Bakthavatsalam D, Meijer HJ, Noegel AA, Govers F. Novel phosphatidylinositol phosphate kinases with a G-protein coupled receptor signature are shared by Dictyostelium and Phytophthora. Trends Microbiol 2006; 14:378 - 382