Abstract
COMATOSE (CTS), the plant homologue of Adrenoleukodystrophy protein, is a full length ABC transporter localised in peroxisomes. In a recent article, we reported that the two nucleotide binding domains of CTS are not functionally equivalent in vivo. Mutations in conserved residues in the Walker A (K487A) and B (D606N) motifs of NBD1 resulted in a null phenotype, whereas identical mutations in the equivalent residues in NBD2 (K1136A and D1276N) had no detectable effect.1 In order to study the effect of these mutations on the ATPase activity of the nucleotide binding domains, we cloned and expressed the isolated NBDs as maltose binding protein (MBP) fusion proteins. We show that ATPase activity is associated with the isolated MBP-NBDs. However, mutations of amino acids located in conserved motifs did not result in striking reduction in activity despite well characterized roles in ATP binding and hydrolysis. We urge caution in the interpretation of results obtained from the study of isolated NBD fusions and their extrapolation to the mechanism of ATP hydrolysis in ABC transporter proteins.
In Vitro Purification and ATPase Activity of Isolated MBP-NBDs
Understanding the ATP hydrolysis mechanism of nucleotide binding domains and their role in the transport of molecules by eukaryotic ABC transporters has proved difficult due to the necessity of purifying and reconstituting the full length transporters in a functional manner.Citation3,Citation4 The use of isolated nucleotide binding domains fused to a tag and expressed in bacteria has often been the method of choice for tackling this problem.Citation5–Citation8
In vivo data suggest CTS NBD1 plays a critical role in function whereas NBD2 is dispensable. This is consistent with the substitution of the switch motif His of the H-loop with Q in CTS NBD2, which would imply reduced ATPase activity as seen in the Transporter for Antigen Presentation (TAP) and the haemolysin B transporter (HlyB).Citation9–Citation11 We tested this hypothesis by cloning both NBD1 and NBD2 fused to either an N-terminal His10-tag (pET16b, Novagen), an N-terminal His6-tag (pET28b, Novagen) or N-terminal MBP-tag (pMal-c2x, NEB) and expressing them in E. coli. In contrast to His-tagged fusion proteins, which were poorly expressed and found mostly in inclusion bodies, MBP-tagged NBDs were soluble, expressed at a high level and could be highly purified by amylose affinity chromatography (). ATPase assays of purified MBP-NBD1 and MBP-NBD2 showed that ATPase activity was associated with both NBD1 and NBD2 fusion proteins (, black squares, top and lower respectively), but not with MBP (, open circles). ATPase activity was comparable with that reported for several previously isolated NBDs.Citation6,Citation12,Citation13
Point mutations in the Walker motifs (corresponding to the mutations studied in vivo) were introduced by site-directed mutagenesis and the resulting purified proteins were tested in a similar manner (). All residues mutated are suggested to be crucial for ATP binding and hydrolysis.Citation2 Similarly, due to the loss of the essential histidine of the H-loop, H636Q should have a reduced ATPase activity, whereas Q1306H would be expected to have increased activity.Citation10,Citation11 However, despite a slight reduction in ATPase activity of D606N and a surprising increase for D1276N, no striking differences in the ATPase activity of the mutants compared to the wild-type were found. This was also observed for the point mutants G503E, C631Y (both of which impair CTS function in vivo),Citation1 E607Q, in which the “catalytic carboxylate” is replaced with glutamine and the double mutants K487A/E607Q and D606N/E607Q (data not shown).
Oligomeric State of MBP-NBD Fusion Proteins
The retention of ATPase activity for all mutants in vitro suggested the potential presence of contaminating activity. Chaperones, which often co-purify with misfolded and/or overexpressed proteins, could thus represent potential candidates because they often possess ATPase activity to release proteins after they are folded. Immunoblotting showed that low levels of GroEL () and DnaK (not shown) were present in the purified protein fractions. However, similar amounts of chaperones were found in the MBP fraction compared to MBP-NBD fractions. Since MBP alone exhibited negligible ATPase activity (), neither GroEL nor DnaK contamination can account for the high ATPase activities found for WT and mutant proteins.
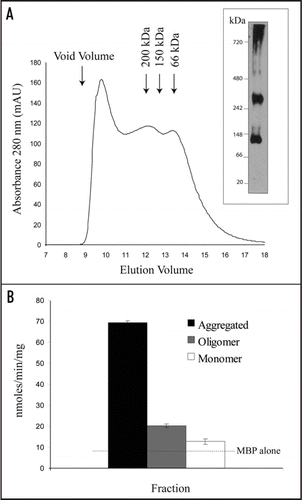
In order to purify the MBP-NBDs further, the amylose-purified fractions were subjected to gel filtration chromatography (). MBP-NBD1 was present in three different forms: a monomer (∼70 kDa), a higher oligomer (>200 kDa) and an aggregated form that was found close to the void volume after gel filtration. This aggregated form did not penetrate a native gel (, right). Aggregation has been reported for other MBP-NBD fusions.Citation6,Citation14 ATPase activity assays were performed using same amount of MBP-NBD1 from each fraction. The aggregated fraction always had the highest ATPase activity, possibly reflecting a highest degree of contamination by unknown ATPases. The monomeric fraction had only a background activity similar to MBP fractions (not shown), which is consistent with the requirement for NBD dimerization to produce ATPase activity.Citation15 When the oligomeric fractions were tested, again no obvious differences were found between WT MBP-NBD and the corresponding mutants. Mixing purified NBD1 and NBD2 did not result in formation of heterodimers or increase in ATPase activity (not shown).
Conclusions
Many authors investigate the activities of given NBDs by cloning and expressing them as fusion proteins. This is an apparently convenient and rapid way to determine the relative ATP binding and hydrolysis constants for a particular protein. Point mutations exhibiting reduced activity have been described,Citation16,Citation17 but mutations greatly reducing the ATPase activity (>80%) of an MBP-NBD fusion protein have only rarely been reported. In our case, the K487A mutation introduced in the full length CTS had a null phenotype in vivoCitation1 and about 80% reduced ATPase activity in the context of the full length protein expressed in a heterologous system (Nyathi Y and Baker A, unpublished), whereas the same mutation introduced into isolated MBP-NBD1 retained full activity ().
An unknown contaminating ATPase activity that co-purified with the MBP-NBD fusions but not with MBP alone could explain this difference, although the major E. coli chaperones GroEL and DnaK could be excluded (). Another possibility is that the choice of domain boundaries used in the NBD1 and NBD2 constructs conferred non-physiological/unusual folding on the proteins, resulting in an ATPase activity independent of the requirement for residues that are normally essential within the native context. Neither of these scenarios is easy to prove or refute. Caution should thus be taken when interpreting ATPase activities of MBP-NBD fusions in vitro.
Abbreviations
| NBD | = | nucleotide binding domain |
| MBP | = | maltose binding protein |
Figures and Tables
Figure 1 Purification and ATPase activity of MBP-NBD variants. (A) E. coli strain BL21Gold cells expressing the various constructs were disrupted in column buffer (20 mM Tris pH 7.4, 200 mM NaCl, 1 mM EDTA) by sonication and the lysate clarified by centrifugation. MBP fusion proteins were purified by amylose affinity chromatography and eluted in 20 mM Tris pH 7.4, 200 mM NaCl, 10 mM Maltose. 1 µg of each purified protein was separated by SDS-PAGE and stained with Coomassie Blue R250. NBD1 comprises amino acids 442 to 685 and NBD2 comprises amino acids 1091 to 1337 delimited by disordered regions present in CTS modeled on homology with Sav1866 (2HYD.pdb) as a structure template.Citation1 (B) Purified proteins were incubated in 50 mM Tris pH 7.4, 0.15 mM NH4Cl, 5 mM MgSO4 in a 10 µl final volume. Reaction was started by the addition of ATP and incubated at 37°C for 30 min. Phosphate release was measured by the method of Chifflet et al. 1988.Citation18,Citation19 Data are means ± SD of the mean and are representative of 3 independent experiments. (C) Equivalent amounts of purified proteins were loaded on a SDS gel and Coomassie stained (left) or transferred onto a nitrocellulose membrane and blotted with an anti-GroEL antibody (right).
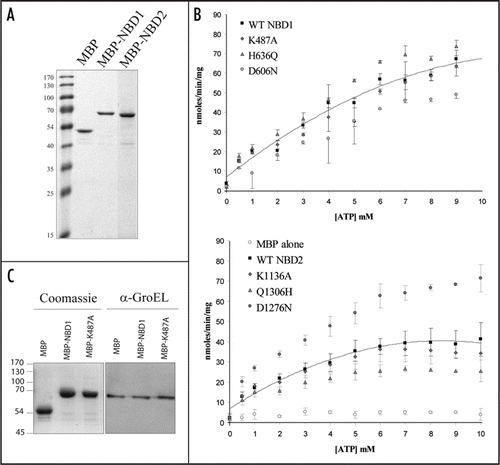
Figure 2 MBP-NBD purify as a mixture of different forms. (A) Amylose-purified MBP-NBD1 fractions were combined, concentrated and loaded onto a Superose 6 column (A, left) or a blue native gel (A, right). The void volume on the gel filtration chromatography is indicated by an arrow. The native gel was transferred onto a PVDF membrane and immunoblotted with anti-CTS antibody. (B) ATPase activities of the three fractions. Equal amounts of protein were assayed, containing the same amount of MBP-NBD1. The dotted line shows the level of activity associated with MBP alone.
Acknowledgements
This work was funded by the Biotechnology and Biology Research Council (BBSRC) grants P19769, P19770, BB/F007299/1 and BB/F007108/1. Rothamsted Research receives grant aided support from the BBSRC of the UK.
Addendum to:
References
- Dietrich D, Schmuths H, De Marcos Lousa C, Baldwin JM, Baldwin SA, Baker A, et al. Mutations in the Arabidopsis peroxisomal ABC transporter COMATOSE allow differentiation between multiple functions in planta: Insights from an allelic series. Mol Biol Cell 2009; 20:530 - 543
- Frelet A, Klein M. Insight in eukaryotic ABC transporter function by mutation analysis. FEBS Lett 2006; 580:1064 - 1084
- Evans GL, Ni B, Hrycyna CA, Chen D, Ambudkar SV, Pastan I, et al. Heterologous expression systems for P-glycoprotein: E. coli, yeast and baculovirus. J Bioenerg Biomembr 1995; 27:43 - 52
- Morita M, Kurisu M, Kashiwayama Y, Yokota S, Imanaka T. ATP-binding and -hydrolysis activities of ALDP (ABCD1) and ALDRP (ABCD2), human peroxisomal ABC proteins, overexpressed in Sf21 cells. Biol Pharm Bull 2006; 29:1836 - 1842
- Berridge G, Walker JA, Callaghan R, Kerr ID. The nucleotide-binding domains of P-glycoprotein: Functional symmetry in the isolated domain demonstrated by N-ethylmaleimide labelling. Eur J Biochem 2003; 270:1483 - 1492
- Wang C, Castro AF, Wilkes DM, Altenberg GA. Expression and purification of the first nucleotide-binding domain and linker region of human multidrug resistance gene product: comparison of fusions to glutathione S-transferase, thioredoxin and maltose-binding protein. Biochem J 1999; 338:77 - 81
- Stratford FL, Ramjeesingh M, Cheung JC, Huan LJ, Bear CE. The Walker B motif of the second nucleotide-binding domain (NBD2) of CFTR plays a key role in ATPase activity by the NBD1–NBD2 heterodimer. Biochem J 2007; 401:581 - 586
- Cheung JC, Kim Chiaw P, Pasyk S, Bear CE. Molecular basis for the ATPase activity of CFTR. Arch Biochem Biophys 2008; 476:95 - 100
- Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J 2005; 24:1901 - 1910
- Procko E, Ferrin-O'Connell I, Ng SL, Gaudet R. Distinct structural and functional properties of the ATPase sites in an asymmetric ABC transporter. Mol Cell 2006; 24:51 - 62
- Ernst R, Koch J, Horn C, Tampé R, Schmitt L. Engineering ATPase activity in the isolated ABC cassette of human TAP1. J Biol Chem 2006; 281:27471 - 27480
- de Wet H, Mikhailov MV, Fotinou C, Dreger M, Craig TJ, Vénien-Bryan C, et al. Studies of the ATPase activity of the ABC protein SUR1. FEBS J 2007; 274:3532 - 3544
- Zingman LV, Hodgson DM, Bienengraeber M, Karger AB, Kathmann EC, Alekseev AE, et al. Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J Biol Chem 2002; 277:14206 - 14210
- Ko YH, Thomas PJ, Delannoy MR, Pedersen PL. The cystic fibrosis transmembrane conductance regulator. Overexpression, purification and characterization of wild type and delta F508 mutant forms of the first nucleotide binding fold in fusion with the maltose-binding protein. J Biol Chem 1993; 268:24330 - 24338
- Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell 2002; 10:139 - 149
- de Wet H, Proks P, Lafond M, Aittoniemi J, Sansom MS, Flanagan SE, et al. A mutation (R826W) in nucleotide-binding domain 1 of ABCC8 reduces ATPase activity and causes transient neonatal diabetes. EMBO Rep 2008; 9:648 - 654
- Procko E, Gaudet R. Functionally important interactions between the nucleotide-binding domains of an antigenic peptide transporter. Biochemistry 2008; 47:5699 - 5708
- Baginski ES, Foà PP, Zak B. Microdetermination of inorganic phosphate, phospholipids and total phosphate in biologic materials. Clin Chem 1967; 13:326 - 332
- Chifflet S, Torriglia A, Chiesa R, Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal Biochem 1988; 168:1 - 4