Abstract
Gap junctions, through their constitutive proteins, connexins (Cx), are involved in several processes including regulation of cellular proliferation, tissue differentiation, homeostasis and neoplasic transformation. Internalization of the gap junction plaque to form annular gap junction is a dynamic process which present similarities with endocytosis and participates in the control of gap junction coupling. Cx43 exhibits dynamic trafficking that needs sequential implication of a large number of protein partners. We have recently shown that ZO-1 localized in both sides of the gap junction plaque was restricted to one side during internalization. The dissociation between ZO-1 and Cx43 particularly occurred on the face where c-Src specifically associated with Cx43, and was abnormally accelerated in response to a carcinogen. In this addendum we summarize and further discuss these results.
Gap junctions and connexins play an essential role in cell growth and differentiation and alteration of their expression has been associated with many diseases and is a typical feature of most tumor cells.Citation1,Citation2 A critical and long-standing question in gap junction biology is how junctional communication is controlled in physiological situation or altered under pathophysiological stimuli. The extent to which cells are functionally coupled by gap junction channels depends on a multiplicity of regulatory mechanisms (gene transcription, translational and post translational modification of connexin…).Citation3 Trafficking of Cx to the plasma membrane to form gap junction plaque, stability and internalization of these structures are also essential steps in the control of Cx function that depends upon the presence of protein partners.Citation4
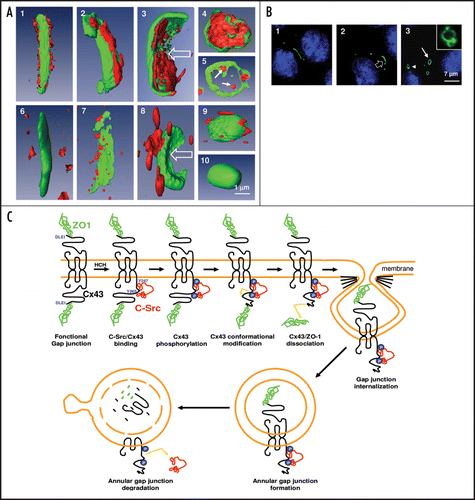
Zonula occludens 1 (ZO-1) a member of the membrane-associated guanylate kinase (MAGUK) is the major Cx-interacting protein. Cx43 interacts with ZO-1 through its C-terminal region that binds the second PDZ domain of ZO-1.Citation5,Citation6 ZO-1 also associates with other Cxs, e.g. Cx31.9,Citation7 Cx36,Citation8 Cx45,Citation9,Citation10 Cx46 and Cx50.Citation11 ZO-1 is required for localization of Cx43 into gap junction plaques,Citation8 but its presence does not appear essential for the formation of functional channels, since Cx43 constructs that lack the C-terminal region, at the site of ZO-1 binding, are able to form gap junction channels and plaques.Citation12–Citation14 In addition, it has been suggested that Cx43/ZO-1 interactions facilitate gap junction stabilization through cytoskeletal anchoring,Citation6,Citation15,Citation16 control the size of the Cx43-containing gap junctional plaque,Citation17 and participate in Cx43 internalization in several cell types, including cardiomyocytes,Citation18 astrocytesCitation15 and Sertoli cells.Citation19 However, if some studies described a potential dissociation of ZO-1 from the Cx43 gap junction plaque during the time-course of internalization,Citation15 others reported that both partners could remain associated in annular gap junction, the degradative form of gap junction plaque.Citation19 We have shown for the first time that during this process of invagination, a molecular reorganization occurred around Cx43 based gap junction plaqueCitation20 as confirmed by three-dimensional Amira reconstruction (). Indeed, our data revealed that ZO-1, which was first present on both sides of the plaque, was further only located on the internal region of the invaginating Cx43-GFP gap junction plaque to finally be found inside the newly formed annular gap junction. In addition, the degradative annular structure moved from the plasma membrane toward the Golgi region for final lysosomal degradation (, arrowhead and inset). During the same time the internal ZO-1 labeling progressively disappeared (, compare 4 to 5), suggesting an internal degradation process of this protein.
Another interesting observation is that, during gap junction plaque endocytosis, ZO-1 was only disrupted on one side of the gap junction plaque corresponding to ZO-1 presence in one of the two communicative and adjacent cells. ZO proteins have been proposed to be scaffolding proteins that could link tight junctions to the actin cytoskeleton through a direct interaction with actin or through additional protein interactions.Citation21 Thus from our data it is tempting to hypothesize that the disrupted ZO-1 presence in one cell leads to altered stabilization of the gap junction plaque equilibrium between the two adjacent cells, which could favor the internalization of the plaque.
There is now evidence that modified interaction with other Cx protein partners could reduce Cx43/ZO-1 association and consequently gap junctional intercellular communication. Indeed by interacting with Cx43 the tyrosine c-Src kinase is involved in both, the dissociation of the Cx43/ZO-1 complex,Citation22 and the downregulation of gap junctional cell-cell communication.Citation15,Citation23,Citation24 Such a process has been clearly characterized after intracellular acidosis in astrocytes by ischemia or hypoxia.Citation15,Citation25 However, the molecular interactions between Cx43 and these two partners, which could drive Cx43 endocytosis in response to carcinogensCitation26–Citation28 were unknown. In the course of a better understanding of the Cx43 partners involved in this process, we reported that c-Src-mediated dissociation of ZO-1 from one side of the plaque initiates gap junction endocytic internalization, and that this process can be markedly amplified in response to HCH, a non genomic carcinogenCitation20 (). We further demonstrated that the modification of the Cx43/c-Src/ZO-1 complex was subsequent to a rapid recruitment of c-Src to the plasma membrane, activation of c-Src, and efficient inhibition of gap junctional coupling. Since c-Src is known to be overexpressed and functionally upregulated in many types of human cancer,Citation29 we speculated that c-Src, by altering the stabilization of the gap junction plaques, could conduce to severe disruption of cell-cell communication that may lead to tumor progression. Altogether our data demonstrate that membranous recruitment of c-Src not only induces closure of gap junction channel as previously reported,Citation15,Citation25 but can also stimulate gap junction internalization.
In conclusion, the results described in our recent studyCitation20 and discussed here allow postulating an innovating model of molecular reorganization () between Cx43 and two of its partners, c-Src and ZO-1, during gap junction plaque endocytosis. The possibility to apply this mechanistic endocytic model to other membranous proteins, unable to form large visualized structures, could be hypothesized.
Figures and Tables
Figure 1 (A) Carcinogen exposure induces gap junction plaque internalization that is associated with the increased interaction of c-Src with Cx43, decreased association between ZO-1. The three-dimensional Amira analysis of the altered interactions between Cx43 (green fluorescence) and ZO-1 (red fluorescence) during gap junction plaque internalization is summarized in upper panels. The lower panels illustrate the modified association between c-Src (red fluorescence) and Cx43 (green fluorescence) during the same time-period. Panel A5 represents the internal annular gap junction degradation of ZO-1 and panel A10 illustrates the disappearance of c-Src from this structure at the same period. (B) Identification of the different phases of gap junction plaque endocytosis analyzed by meaning of Cx43 tagged with green fluorescent protein (GFP). Left: Cx43-GFP gap junction plaque observed between two adjacent cells. Middle: invagination of the structure within one cell. Right: formation of an annular gap junction, which is present at the beginning of the endocytosis phase near the plasma membrane (arrow) and afterwards in the nuclear region around the nucleus (arrowhead). The inset represents a degradative form of annular gap junction. (C) Schematic representation of the molecular mechanisms by which ZO-1 and c-Src interact with Cx43 during gap junction plaque endocytosis. In control cells, Cx43 is associated with ZO-1. After HCH exposure, activated c-Src bind to Cx43 and induced Cx43 phosphorylation on tyrosine residues as suggested.Citation30 These modifications could be involved in Cx43/ZO1 dissociation in the gap junction side that binds c-Src, and induce gap junction internalization on the side of the gap junction plaque in which Cx43/ZO-1 association was probably affected in concert with other Cx43 protein partners (clathrin, actin…). Then annular gap junctions that contain ZO-1 inside and c-Src outside of the vesicle are degraded during annular gap junction trafficking from the plasma membrane to lysosomal area close to nuclei giving rise to disappearance of both proteins from their original position.
Addendum to:
References
- Leithe E, Sirnes S, Omori Y, Rivedal E. Downregulation of gap junctions in cancer cells. Crit Rev Oncog 2006; 12:225 - 256
- Pointis G, Fiorini C, Gilleron J, Carette D, Segretain D. Connexins as precocious markers and molecular targets for chemical and pharmacological agents in carcinogenesis. Curr Med Chem 2007; 14:2288 - 2303
- Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta 2005; 1711:215 - 224
- Hervé JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol 2007; 94:29 - 65
- Giepmans B, Moolenaar W. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol 1998; 8:931 - 934
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 1998; 273:12725 - 12731
- Nielsen PA, Beahm DL, Giepmans BN, Baruch A, Hall JE, Kumar NM. Molecular cloning, functional expression and tissue distribution of a novel human gap junction-forming protein, connexin-31.9. Interaction with zona occludens protein-1. J Biol Chem 2002; 277:38272 - 38283
- Li X, Olson C, Lu S, Kamasawa N, Yasumura T, Rash JE, Nagy JI. Neuronal connexin36 association with zonula occludens-1 protein (ZO-1) in mouse brain and interaction with the first PDZ domain of ZO-1. Eur J Neurosci 2004; 19:2132 - 2146
- Laing JG, Manley-Markowski RN, Koval M, Civitelli R, Steinberg TH. Connexin45 interacts with zonula occludens-1 and connexin43 in osteoblastic cells. J Biol Chem 2001; 276:23051 - 23055
- Kausalya PJ, Reichert M, Hunziker W. Connexin45 directly binds to ZO-1 and localizes to the tight junction region in epithelial MDCK cells. FEBS Lett 2001; 505:92 - 96
- Nielsen PA, Baruch A, Shestopalov VI, Giepmans BN, Dunia I, Benedetti EL, et al. Lens connexins alpha3Cx46 and alpha8Cx50 interact with zonula occludens protein-1 (ZO-1). Mol Biol Cell 2003; 14:2470 - 2481
- Fishman GI, Moreno AP, Spray DC, Leinwand LA. Functional analysis of human cardiac gap junction channel mutants. Proc Natl Acad Sci USA 1991; 88:3525 - 3529
- Dunham B, Liu S, Taffet S, Trabka-Janik E, Delmar M, Petryshyn R, et al. Immunolocalization and expression of functional and nonfunctional cell-to-cell channels from wild-type and mutant rat heart connexin43 cDNA. Circ Res 1992; 70:1233 - 1243
- Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science 1999; 283:1176 - 1180
- Duffy H, Ashton A, O'Donnell P, Coombs W, Taffet S, Delmar M, Spray D. Regulation of connexin43 protein complexes by intercellular acidification. Circ Res 2004; 94:215 - 222
- Akoyev V, Takemoto DJ. ZO-1 is required for protein kinase C gamma-driven disassembly of connexin 43. Cell Signal 2007; 19:958 - 967
- Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res 2002; 90:317 - 324
- Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 2005; 16:5686 - 5698
- Segretain D, Fiorini C, Decrouy X, Defamie N, Prat JR, Pointis G. A proposed role for ZO-1 in targeting connexin 43 gap junctions to the endocytic pathway. Biochimie 2004; 86:241 - 244
- Gilleron J, Fiorini C, Carette D, Avondet C, Falk MM, Segretain D, et al. Molecular reorganization of Cx43, ZO-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J Cell Sci 2008; 121:4069 - 4078
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 2008; 1778:660 - 669
- Toyofuku T, Akamatsu H, Zhang T, Kuzuya T, Tada M, Hori M. C-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem 2001; 276:1780 - 1788
- Postma FR, Hengeveld T, Alblas J, Giepmans BNG, Zondag GCM, Jalink K, et al. Acute loss of cell-cell communication caused by G protein-coupled receptors: a critical role for c-Src. J Cell Biol 1998; 140:1199 - 1209
- Sorgen P, Duffy H, Sahoo P, Coombs W, Delmar M, Spray D. Structural changes in carboxyl terminus of the gap junction protein connexin-43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem 2004; 279:54695 - 54701
- Li W, Hertzberg EL, Spray DC. Regulation of connexin43-protein binding in astrocytes in response to chemical ischemia/hypoxia. J Biol Chem 2005; 280:7941 - 7948
- Defamie N, Mograbi B, Roger C, Cronier L, Malassine A, Brucker-Davis F, et al. Disruption of gap junctional intercellular communication by lindane is associated with aberrant localization of connexin43 and zonula occludens-1 in 42GPA9 Sertoli cells. Carcinogenesis 2001; 22:1537 - 1542
- Mograbi B, Corcelle E, Defamie N, Samson M, Nebout M, Segretain D, et al. Aberrant Connexin 43 endocytosis by the carcinogen lindane involves activation of the ERK/mitogen-activated protein kinase pathway. Carcinogenesis 2003; 24:1415 - 1423
- Fiorini C, Gilleron J, Carette D, Valette A, Tilloy A, Chevalier S, et al. Accelerated internalization of junctional membrane proteins (connexin 43, -cadherin and ZO-1) within endocytic vacuoles: an early event of DDT carcinogenicity. Biochim Biophys Acta 2008; 1778:56 - 67
- Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 2002; 1602:114 - 130
- Pahujaa M, Anikin M, Goldberg GS. Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells. Exp Cell Res 2007; 313:4083 - 4090