Abstract
Classical cadherins are a group of Ca++ dependent transmembrane cell adhesion molecules, mostly known for their ability to perform homophylic interactions with like-cadherin molecules on the surface of neighboring cells. Over the past decade, many studies have also established cadherins as key players of intracellular signaling events by modifying the activity of Rho GTPases, members of the Wnt signaling pathway, and receptor tyrosine kinases. Given the utility of these molecules, it is not surprising that they play multiple roles during different embryological and adult processes. Yet, these activities have been primarily tied to their full-length molecules. And, while the activity of full-length molecules is undoubtedly an essential part of how cadherins perform in vivo, it is becoming increasingly evident that the proteolytic fragments of these molecules may also play a role. This is an exciting development because proteolysis of cadherins was previously thought to be a simple clearing-mechanism meant to regulate the levels of cadherin molecules on the cell-surface.
Here, we will further discuss our recent findings by McCusker and colleagues, showing that both N-terminal and C-terminal fragments of Cadherin-11 retain biological activity in Xenopus embryos. We will also review the current literature demonstrating that both the extracellular and intracellular fragments of other classical cadherins are capable of activating certain signaling events tied to Epithelial to Mesenchymal Transitions (EMTs), cell survival, cell proliferation, and cell migration.
Proteolysis of Classical Cadherins
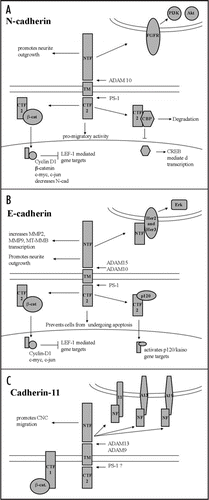
Complete processing of a full-length cadherin molecule involves multiple proteases. It is generally accepted that the extracellular region is processed first, and that multiple metalloproteases containing a disintegrin domain (ADAM), Matrix Metalloproteases (MMPs), and other transmembrane proteases can perform this event.Citation1–Citation8 This initial cleavage results in the shedding of the extracellular N-terminal fragment (NTF), and the generation of a first C-terminal fragment (CTF1) that contains the transmembrane and cytoplasmic domains of the molecule. In many cases CTF1 is further processed by the Presenilin-1 (PS-1) complex in the juxta-membrane region, releasing the cytoplasmic domain (CTF2) along with any associated proteins.Citation9–Citation12 Among the classical cadherins, there are multiple examples showing that the NTF, CTF1 and CTF2 can each perform diverse biological activities ().
Biological Activities of Shed Cadherin Fragments
In the recent publication by McCusker and colleagues, we showed that cadherin-11 cleavage by ADAM metalloproteases (ADAM9, and 13) was essential for Cranial Neural Crest (CNC) migration in vivo.Citation6 One likely purpose of this processing is to control the overall cell-adhesion levels mediated by the full-length cadherin-11 molecules. Yet, we have also discovered that the NTF itself can promote migration in vivo. In Xenopus embryos, CNC migration is inhibited when full-length cadherin-11 is overexpressed or when ADAM9, 13 and 19 expression are knocked down. This phenotype can be rescued by overexpressing the cadherin-11 NTF. We suspect that the cadherin-11 NTF acts as an antagonist of homophylic interactions since we have shown that it binds to full-length cadherin-11 in cell culture.Citation6 Interestingly, we have also found that the NTF can bind to select members of the ADAM family (McCusker and Alfandari, unpublished observations), regardless of their ability to process full-length cadherin-11. Future work will determine if this interaction affects the function of these ADAMs and if this interaction plays a physiological role in the embryo.
The NTFs of both N-cadherin and E-cadherin have also been shown to have biological activities. Endogenous N-cadherin NTF has been detected in vivo in the extracellular matrix (ECM) surrounding denervated muscle fibers, as well as in embryonic retinal tissue.Citation13,Citation14 N-cadherin NTF that is associated with the ECM may perform an important biological function in cell adhesion and neurite outgrowth, as immobilized NTF promotes both of these activities in cell culture.Citation14,Citation15 N-cadherin NTF can also associate with and activate FGF receptors, resulting in the activation of PI3K and Akt, and decreasing the levels of apoptosis.Citation16 Likewise, E-cadherin NTF was shown to bind to, and activate the human ErbB receptors, Her2 and Her3, leading to the activation of downstream signaling that results in cell migration and cell proliferation in cell culture.Citation7 Therefore, the NTFs of these classical cadherins can affect cell adhesion properties, as well as bind to and activate cell-surface receptors.
Signaling Through the CTFs
The cadherin fragment signaling capabilities do not end with the extracellular region. The CTFs of classical cadherins can also influence intracellular signaling events by interacting with molecules involved in multiple pathways. For example, the N-cadherin CTF2 associates with CREB Binding Protein (CBP), targets it for degradation, and prevents its ability to promote CREB-mediated transcription in cell culture.Citation10 Marambaud and colleagues have speculated that this activity may be important for neuronal growth and survival.Citation10 In addition, Ferber and colleagues have shown that the E-cadherins CTF2 can activate the expression of Wnt-related genes that are involved in cell proliferation and differentiation in cell culture.Citation9 In this instance, E-cadherins CTF2 binds to p120 catenin, translocates to the nucleus, and can activate transcription of Wnt target genes by blocking the repressor Kaiso.Citation9
The CTFs of N-cadherin, E-cadherin and cadherin-11 all associate with the Wnt signaling molecule, β-catenin. N-cadherin and E-cadherin CTF2 both interact with β-catenin, prevent its degradation and relocate it to the nucleus to promote gene transcription.Citation4,Citation5,Citation10,Citation11,Citation17,Citation18 As a result, the CTF2 of N-cadherin and E-cadherin can both effect the transcription of a number of downstream targets such as cyclin D1, c-myc and c-jun, and can enhance cell behaviors such as cell proliferation and cell migration in cell culture and in vivo.Citation5,Citation11,Citation17,Citation19 Yet, it is not entirely resolved if CTF2 alone or the CTF2/β-catenin complex is responsible for all of these processes.
On the other hand, cadherin-11s interaction with β-catenin appears to deviate somewhat from what has been established with the other classical cadherins. While we can detect a small cadherin-11 CFT2-sized fragment in embryos, we have not yet established if it complexes with β-catenin.Citation6 However, we have shown that the cadherin-11 CTF1 maintains its interaction with β-catenin, and does not stimulate the activation of the transcriptional targets described above.Citation6 In the case of CNC migration we suspect that is an important detail, since β-catenin signaling can block CNC migration if activated exogenously during this process.Citation20
Teasing Apart the Role of Full-Length vs. Cadherin Fragment Affect on Signaling
One of the future objectives of our laboratory is to determine if endogenous cadherin fragments have a physiological function. As we pursue this goal we are challenged with a major difficulty: how do we decipher between the activities of a full-length cadherin molecule and that of its cleavage fragment?
For example, in order to see if a cleavage fragment elicits a certain phenotype others and we often utilize recombinant technology to express peptides meant to mimic the NTFs and CTFs of a cadherin. In this instance, it is difficult to determine if phenotypes observed with overexpressed fragments are specifically caused by their “fragment” properties, or their ability to mimic the full-length molecule. It is probable that the structure of endogenous and recombinant “fragments” varies significantly from the uncleaved peptides, making them molecularly distinguishable. However, these peptides have been shown to interact with many of the same binding partners of the full-length molecule, suggesting that the full-length and cleaved peptides share significant similarities. In addition, it also unclear if the physiological level of a cleavage fragment is enough to promote the same cell behaviors observed when overexpressing recombinant cleavage fragments. So, while the use of recombinant proteins is a useful and essential step to help realize the signaling potential of these fragments, we cannot be sure that the endogenous fragments are performing in the same manner.
The use of chemical inhibitors to block protease activity is another tool that we have used to help determine if the cleavage of a cadherin is important for a physiological process. The advantage of using these inhibitors is that cadherin cleavage can be blocked temporally. However, it is unlikely that they will help us resolve if signaling events promoted by the endogenous fragments are important for a specific cellular behavior. One reason for this is that we could not be sure that a phenotype we observed was a result of the inability to generate a cleavage fragment and its subsequent signaling events, or the inability to process (remove) the full-length molecule. But more importantly, these inhibitors block multiple proteases, and any phenotype we observe could easily be attributed to any of their proteolytic targets.
Perhaps the main source of the experimental difficulties described above is due to the fact that cadherins have important cellular functions before they are processed. Therefore, we must be careful not to interfere with the activity of the full-length cadherin while studying possible signaling activities of its cleavage fragments. One possibility is to generate antibodies that recognize a cryptic site revealed on the cleavage fragment upon processing. This would allow us to see exactly where and when the fragments are generated during a particular biological process. It is also possible that one such antibody could block the “function” of NTFs and would be extremely useful in deciphering a role for these molecules in vivo. Alternately, the generation of small, “function blocking” peptides that are expressed under inducible promoters would be a useful tool for understanding the roles of endogenous CTFs. These peptides would make it possible to temporally block CTF activity in vivo.
Another tool that could further decipher the role of cadherin cleavage would be to generate a mutant in which the cleavage site has been replaced by a sequence cleaved by an exogenous protease. This mutant could be expressed in embryos lacking the wild type cadherin and perform all the functions of the full-length protein but would be unable to be processed by its natural protease. In return, the exogenous protease could be provided at a define time to observe the change in cell behavior. The two main technical difficulties of such an experiment is (1) to find a sequence that would not be cleaved by the natural protease which is always difficult with ADAM and (2) to identify an exogenous protease whose expression would not adversely affect embryo development.
To conclude, it is clear that we will need to come up with creative solutions as we determine the physiological relevance of cadherin cleavage fragments. Yet, as new tools and technologies emerge, we will be able to resolve these issues.
Abbreviations
| ADAM | = | |
| MMP | = | |
| NTF | = | |
| CTF | = | |
| PS-1 | = | |
| CNC | = | |
| ECM | = | |
| FGF | = | |
| PI3K | = | |
| ErbB | = | |
| Her2 and Her3 | = | |
| CREB | = | |
| CBP | = |
Figures and Tables
Figure 1 The extracellular (NTF) and intracellular (CTF) cleavage fragments of classical cadherins retain biological activities. (A) N-cadherin NTF and CTF can interact with the FGFR, β-catenin and CREB signaling molecules. These interactions can lead to transcriptional activation or inhibition, and can promote cell migration. (B) E-cadherin cleavage fragments can interact with EGF receptors, β-catenin and p120 catenin signaling molecules. These fragments can protect cells from apoptosis, support cell migration and promote the transcription of a number of genes. (C) Cadherin-11 cleavage fragments can interact with full-length cadherin-11, ADAM13, ADAM19 and β-catenin, and promote cell migration in vivo.
Acknowledgements
The authors would like to thank Russell Neuner, Julian Sosnik and Dr. Hélène Cousin for their helpful comments on the manuscript. Catherine McCusker and Dr. Dominque Alfandari are supported by a grant from USPHS (DE016289) to Dr. Alfandari.
References
- Davies G, Jiang WG, Mason MD. Matrilysin mediates extracellular cleavage of E-cadherin from prostate cancer cells: a key mechanism in hepatocyte growth factor/scatter factor-induced cell-cell dissociation and in vitro invasion. Clin Cancer Res 2001; 7:3289 - 3297
- Hermant B, Bibert S, Concord E, Dublet B, Weidenhaupt M, Vernet T, et al. Identification of proteases involved in the proteolysis of vascular endothelium cadherin during neutrophil transmigration. J Biol Chem 2003; 278:14002 - 14012
- Hunter I, McGregor D, Robins SP. Caspase-dependent cleavage of cadherins and catenins during osteoblast apoptosis. J Bone Miner Res 2001; 16:466 - 477
- Ito K, Okamoto I, Araki N, Kawano Y, Nakao M, Fujiyama S, et al. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene 1999; 18:7080 - 7090
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration and beta-catenin translocation. Proc Natl Acad Sci USA 2005; 102:9182 - 9187
- McCusker C, Cousin H, Neuner R, Alfandari D. Extracellular Cleavage of cadherin-11 by ADAM Metalloproteases Is Essential for Xenopus Cranial Neural Crest Cell Migration. Mol Biol Cell 2008;
- Najy AJ, Day KC, Day ML. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem 2008; 283:18393 - 18401
- Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem 2001; 276:4972 - 4980
- Ferber EC, Kajita M, Wadlow A, Tobiansky L, Niessen C, Ariga H, et al. A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem 2008; 283:12691 - 12700
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, et al. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 2003; 114:635 - 645
- Uemura K, Kihara T, Kuzuya A, Okawa K, Nishimoto T, Ninomiya H, et al. Characterization of sequential N-cadherin cleavage by ADAM10 and PS1. Neurosci Lett 2006; 402:278 - 283
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J 2002; 21:1948 - 1956
- Cifuentes-Diaz C, Nicolet M, Goudou D, Rieger F, Mege RM. N-cadherin expression in developing, adult and denervated chicken neuromuscular system: accumulations at both the neuromuscular junction and the node of Ranvier. Development 1994; 120:1 - 11
- Paradies NE, Grunwald GB. Purification and characterization of NCAD90, a soluble endogenous form of N-cadherin, which is generated by proteolysis during retinal development and retains adhesive and neurite-promoting function. J Neurosci Res 1993; 36:33 - 45
- Utton MA, Eickholt B, Howell FV, Wallis J, Doherty P. Soluble N-cadherin stimulates fibroblast growth factor receptor dependent neurite outgrowth and N-cadherin and the fibroblast growth factor receptor co-cluster in cells. J Neurochem 2001; 76:1421 - 1430
- Lyon CA, Johnson JL, Williams H, Sala-Newby GB, George SJ. Soluble N-Cadherin Overexpression Reduces Features of Atherosclerotic Plaque Instability. Arterioscler Thromb Vasc Biol 2008;
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, et al. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. Embo J 2005; 24:742 - 752
- Sadot E, Simcha I, Shtutman M, Ben-Ze'ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci USA 1998; 95:15339 - 15344
- Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development 2007; 134:491 - 501
- de Melker AA, Desban N, Duband JL. Cellular localization and signaling activity of beta-catenin in migrating neural crest cells. Dev Dyn 2004; 230:708 - 726