Abstract
Catechin is a highly studied but controversial allelochemical reported as a component of the root exudates of Centaurea maculosa. Initial reports of high and consistent exudation rates and soil concentrations have been shown to be highly inaccurate, but the chemical has been found sporadically in root exudates and in soil at much lower levels. Part of the problem of detection and measuring phytotoxicity in natural soils may be due to the confounding effect of soil microbes, and little is known about interactions between catechin and soil microbes. Here we tested the effect of catechin on soil microbial communities and the feedback of these effects to two plant species. We found that catechin inhibits microbial activity in the soil we tested, and by doing so appears to promote plant growth in the microbe-free environment. This is in striking contrast to other in vitro studies, emphasizing the highly conditional effects of the chemical and suggesting that the phytotoxic effects of catechin may be exerted through the microbes in some soils.
Centaurea maculosa, a Eurasian invader in North America, has been reported to release (±)-catechin (hereafter referred as catechin) from its roots,Citation1,Citation2 and this chemical has been shown to be phytotoxic in vitro, in sand cultures and in the field.Citation3–Citation10 A number of other authors have reported phytotoxicity of the (+)-catechin formCitation11–Citation14 and the (-)-catechin form has been reported to inhibit green algae.Citation15 However, other authors have found no phytotoxic effects of forms of catechin or phytotoxicity only at unreasonably high concentrations.Citation1 Furubayashi et al.Citation13 found that phytotoxic effects of (+)-catechin on lettuce (which is a very insensitive species to the chemical)Citation6 were manifest in some soils but not others. Furthermore, the effects of catechin on grasses in field experiments are highly variable among sites.Citation9,Citation10 There is also initial evidence that Centaurea is more allelopathic to North American native species than congeneric European native species in vitroCitation10,Citation16 and in the field,Citation7 and these biogeographic differences have been suggested to be consistent with the “Novel Weapon Hypothesis” (NWH).Citation17,Citation18 This is the idea that some invaders may succeed because they possess unique allelopathic, defense or antimicrobial biochemistry to which naïve natives have not adapted.
Phytotoxicity has been repeatedly demonstrated, but the most controversial aspects of the potential role of catechin as an allelopathic compound are whether or not the roots exude enough catechin to be phytotoxic, and the conflicting reports on soil concentrations; are they high enough to be phytotoxic.Citation19 Early reports of very high catechin concentrations in soilsCitation6,Citation20,Citation21 are not reliable due to sample contamination during analysisCitation19 and the inability to consistently find such high concentrations in later field studiesCitation19,Citation22 (Callaway RM and Vivanco JM, unpublished data). Blair et al.Citation22 reported finding catechin in many soil samples but at low levels, never more than 1 µg g−1, and argued that this concentration could not be phytotoxic. Perry et al.Citation19 using a minimum detection limit of ≈25 µg g−1, detected catechin in only 20 soil samples out of 402, but this was for a set of plants repeatedly measured over a season, and at one point in time all plants at the site were associated with catechin in the soil at a very high mean concentration (650 ± 450 (SD) µg g−1. Pulsed releases of roughly similar concentrations have also occurred in mesocosms with C. maculosa (Schultze M and Paschke M, unpublished data). Extreme variation in detection may be due to pulsed releases, but catechin is highly ephemeral in soil, and applied concentrations of (±)-catechin result in far lower concentrations in solution, sand and soil than calculated from the application rate.Citation9 This may be due to the interaction of the compound with components in the soils and oxidation. However, catechin detection and phytotoxicity may also be affected by soil microbial communities, a component of the system that has been minimally explored.Citation5,Citation23,Citation24
Catechin Phytotoxicity and Soil Microbial Communities
In this Addendum, we report the impact of catechin on soil microbial activity by measuring soil CO2 release, and modification of catechin phytotoxicity in soil free from soil microbes. Levels of nitrogen (N) and phosphorus (P) in soil are influenced by microbial activity and death; we measured levels of these nutrients in catechin-treated soil.
Fifty g soil was placed in each of 9-cm Petri dishes, and treated with appropriate amount of catechin to get final concentrations of 133, 266 or 400 µg catechin/g soilCitation9 (see for methodology). Based on previous workCitation9 () these added concentrations to this particular soil probably created detectable or effective concentrations of >1, 1–5 and 1–40 µg catechin/g soil. It is important to note that bulk soil concentrations of an allelochemical far overestimate the phytotoxic dose because interactions can occur at root-root interfaces; however, these concentrations provide a reasonable place to start. Soil treated with the same amount of water served as controls. Ten seeds of Phalaris minor or Brassica campestris were placed on control or treated soil. Data on root and shoot length were collected after 7 days. Each experiment was replicated six times. Since CO2 release is a good indicator of microbial activity in different soils,Citation25 we measured soil CO2 respiration by chemical titration following Andersen.Citation26 Soil was treated with catechin to achieve added concentrations of 0, 133, 266 or 400 µg catechin/g soil.Citation9 Ten mL 0.1 N NaOH was placed in each 5-cm Petri dish, which was then placed in a chamber (433 cm3) filled with 150 g control or treated soil. Chambers were then immediately covered and care was taken to avoid any loss of CO2. Soil was incubated for 24 h, and experiment was terminated by adding 1 mL of 0.1 N BaCl2 to NaOH. Ten mL of NaOH taken from blanks, controls and treatments was titrated against 0.1 N HCl, and the amount of CO2 released was calculated. Control and treated soils were analyzed for extractable phosphate-P using molybdenum blue method.Citation27 To determine total organic N, soil was digested using the semi-micro Kjeldahl method, and N concentration was determined using the indophenol blue method.Citation27
When soil was sterilized, several concentrations of catechin increased shoot length (; FPhalaris = 12.743, df = 3, 199, p < 0.001; FBrassica = 7.316, df = 3, 229, p < 0.001) and root length (FPhalaris = 10.722, df = 3, 199, p < 0.001; FBrassica = 8.992, df = 3, 229, p < 0.001) and no concentration inhibited the growth of these species. In our previous study, catechin addition to this same soil, but not sterilized, inhibited plant growth at very low detectable concentrations (1.4 ± 1.4 to 36.1 ± 10.2 [1 SE] µg g−1)Citation9, which suggests that in some soils and for some species catechin may need to interact with soil microbial communities to cause plant growth inhibition. Phalaris was inhibited in the initial experiment,Citation9 and to our knowledge the only species in the Brassicaceae tested with catechin is Arabidopsis thaliana, which is highly sensitive.Citation14 We emphasize however, that in other experiments catechin has been shown to be phytotoxic in vitro and, river sand, and sterile sand,Citation9,Citation16 and thus these microbial effects are likely to be just one more component of the conditional effects of this species.
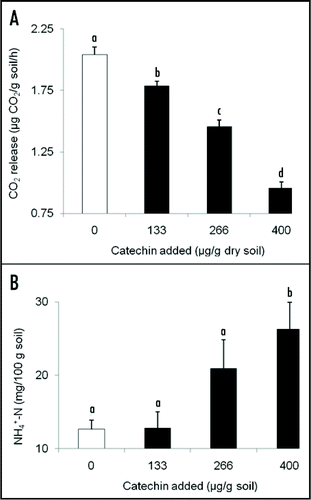
We observed a concentration-dependent decline in CO2 efflux in non-sterile soil treated with catechin (F = 84.254, df = 3, 20, p < 0.0001) () suggesting that catechin killed microbes. (+)-Catechin has inhibitory effects on soil microbial density in vitro and this effect is far stronger on microbes that are found in North American soils than those from European soils.Citation5 No significant differences were observed in the soil concentrations of PO4-P (F =1.591, df = 3, 20, p = 0.223). We observed higher total N levels in soil treated with 400 µg/g soil compared to untreated soil (t = −3.799, P(2-tailed) = 0.004) ( inset), but there is no clear explanation for why suppressing total microbial activity would increase total soil N, unless all microbes but free living N2-fixers were suppressed and N2-fixers were enhanced. Acinetobacter calcoaceticus, which does not fix N, can utilize other forms of catechin as its sole carbon sourceCitation23 and genes involved in the utilization of various forms of catechin have been reported from the genomic DNA of Rhizobium sp. and Bradyrhizobium japonicum USDA 110, both N2-fixers.Citation28,Citation29
Our results indicate that catechin can inhibit general soil microbial activity and that in some soils the role of soil microbial communities may function as either drivers or passengers for catechin phytotoxicity. Either way, these results are a step towards a better understanding of the conditional effects of catechin.
Figures and Tables
Figure 1 Mean (+SE) shoot (black bar) and root (white bar) length of Phalaris minor (A) and Brassica campestris (B) in sterilized soil treated with 0, 133, 266 or 400 µg/g soil. Results from Inderjit et al.Citation9 indicated that these application rates produced detectable concentrations of roughly 0, >1, 1–5 and 1–40 µg catechin/g soil. Shared letters indicate no significant differences among treatments and control as determined by one-way ANOVA with treatment as fixed variable, and post ANOVA Tukey test (p < 0.05).
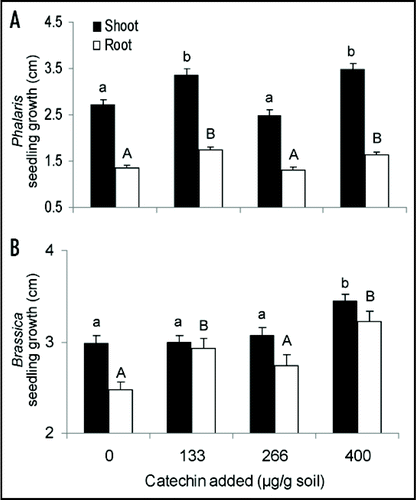
Figure 2 (A) Mean (+SE) CO2 release (µg CO2 released/g soil/h) from soil treated with 0, 133, 266 or 400 µg catechin/g soil. Results from Inderjit et al.Citation9 indicated that these application rates produced detectable concentrations of roughly 0, >1, 1–5 and 1–40 µg catechin/g soil. (B) Mean (+SE) total organic N (mg/100 g dry soil) of soil treated with 0, 133, 266 or 400 µg catechin/g soil. Shared letters indicate no significant differences among treatments and control as determined by one-way ANOVA with treatment as fixed variable, and post ANOVA Tukey test (p < 0.05).
Acknowledgements
Research funding was given by the Department of Science and Technology (DST) and the University of Delhi.
Addendum to:
References
- Blair AC, Hanson BD, Brunk GR, Marrs RA, Westra P, Nissen SJ, Hufbauer RA. New techniques and findings in the study of a candidate allelochemical implicated in invasion success. Ecol Lett 2005; 8:1039 - 1047
- Ridenour WM, Vivanco JM, Feng Y, Horiuchi, Callaway RM. No evidence for tradeoffs: Centaurea plants from America are better competitors and defenders. Ecol Monogr 2008; 78:369 - 386
- Weir TL, Bais HP, Vivanco JM. Intraspecific and interspecific interactions mediated by a phytotoxin, (-)-catechin, secreted by the roots of Centaurea maculosa (spotted knapweed). J Chem Ecol 2003; 29:2379 - 2394
- Weir TL, Bais HP, Stull VJ, Callaway RM, Thelen GC, et al. Oxalate contributes to the resistance of Gaillardia grandiflora and Lupinus sericeus to a phytotoxin produced by Centaurea maculosa. Planta 2006; 223:785 - 795
- Callaway RM, Hierro JL, Thorpe AS. Sax DF, Gaines SD, Stachowicz JJ. Evolutionary trajectories in plant and soil microbial communities: Centaurea invasions and the geographic mosaic of coevolution. Exotic species invasions: insights into ecology, evolution and biogeography 2005; Sunderland, MA Sinauer 341 - 363
- Perry LG, Thelen GC, Ridenour WM, Weir TL, Callaway RM, Paschke MW, et al. Dual role for an allelochemical: (±)-catechin from Centaurea maculosa root exudates regulates conspecific seedling establishment. J Ecol 2005; 93:1126 - 1135
- Thorpe A. Biochemical effects of Centaurea maculosa on soil nutrient cycles and plant communities 2006; Missoula University of Montana PhD Dissertation
- Rudrappa T, Bonsall J, Gallagher JL, Seliskar DM, Bais HP. Root-secreted allelochemical in the noxious weed Phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J Chem Ecol 2007; 33:1898 - 1918
- Inderjit, Pollock JL, Callaway RM, Holben W. Phytotoxic effects of (±)-catechin in vitro, in soil, and in the field. PLoS One 2008; 3:2536; http://dx.doi.org/10.1371/journal.pone.0002536
- Inderjit, Seastedt TR, Callaway RM, Pollock J, Kaur J. Allelopathy and plant invasions: traditional, congeneric and biogeographical approaches. Biol Invas 2008; 10:875 - 890
- Buta JG, Lusby WR. Catechins as germination and growth inhibitors in lespedeza seeds. Phytochemistry 1986; 25:93 - 95
- Iqbal Z, Hiradate S, Noda A, Isojima S, Fujii Y. Allelopathic activity of buckwheat: isolation and characterization of phenolics. Weed Sci 2003; 51:657 - 662
- Furubayashi A, Hiradate S, Fujii Y. Role of catechol structure in the adsorption and transformation reactions of L-Dopa in soils. J Chem Ecol 2007; 33:239 - 250
- Simões K, Du J, Kretzschmar FS, Broeckling CD, Stermitz FS, Vivanco JM, et al. Phytotoxic catechin leached by seeds of the tropical weed Sesbania virgata. J Chem Ecol 2008; 34:681 - 687
- D'Abrosca B, Dellagreca M, Fiorentino A, Isidori M, Monaco P, Pacifico S. Chemical constituents of the aquatic plant Schoenoplectus lacustris: evaluation of phytotoxic effects on the green alga Selenatrum capricornutum. J Chem Ecol 2006; 32:81 - 96
- Callaway RM, Aschehoug ET. Invasive plant versus their new and old neighbors: a mechanism for exotic invasion. Science 2000; 290:521 - 523
- Callaway RM, Ridenour WM. Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2004; 2:436 - 443
- Perry LG, Thelen GC, Ridenour WM, Callaway RM, Paschke MW, Vivanco JM. Concentrations of the allelochemical (+/-)-catechin in Centaurea maculosa soils. J Chem Ecol 2007; 33:2337 - 2344
- Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 2003; 301:1377 - 1380
- Thelen GC, Vivanco JM, Newingham B, Good W, Bais HP, Landres P, et al. Insect herbivory stimulates allelopathic exudation by an invasive plant and the suppression of natives. Ecol Lett 2005; 8:209 - 217
- Blair AC, Nissen SJ, Brunk GR, Hufbauer RA. A lack of evidence for an ecological role of the putative allelochemical (±)-catechin in spotted knapweed invasion success. J Chem Ecol 2006; 32:2327 - 2331
- Arunachalam M, Mohan N, Mahadevan A. Cloning of Acinetobactor calcoaceticus chromosomal region involved in catechin degradation. Microbiol Res 2003; 158:37 - 46
- Thorpe AS, Archer V, Deluca TH. The invasive forb, Centaurea maculosa, increases phosphorus availability in Montana grasslands. Appl Soil Ecol 2006; 32:118 - 122
- Haney RL, Brinton WF, Evans E. Soil CO2 respiration: comparison of chemical titration, CO2 IRGA analysis and the solvita gel system. Ren Agric Food Sys 2008; 23:171 - 176
- Anderson JPE. Page AL, Miller RH, Keeney DR. Soil respiration. Methods of soil analysis: chemical and microbiological properties 1982; Madison, USA American Society of Agronomy and Soil Science Society of America 831 - 871
- Allen SE. Chemical analysis of ecological materials 1989; London Blackwell
- Podila, Kotagiri GK, Santharam S. Cloning of protocatechuate 3,4-dioxygenase genes from Bradyrhizobium japonicum USDA 110. Appl Environ Microbiol 1993; 59:2117 - 2119
- Latha S. Cloning of Rhizobium sp. for catechin oxygenase 1997; University of Madras Chennai, India Ph. D. thesis
- Inderjit, Callaway RM, Vivanco JM. Can plant biochemistry contribute to understanding of invasion ecology?. Trends Plant Sci 2006; 11:574 - 580